Solriamfetol Improves Cognition in Individuals with Excessive Daytime Sleepiness Due to Obstructive Sleep Apnea
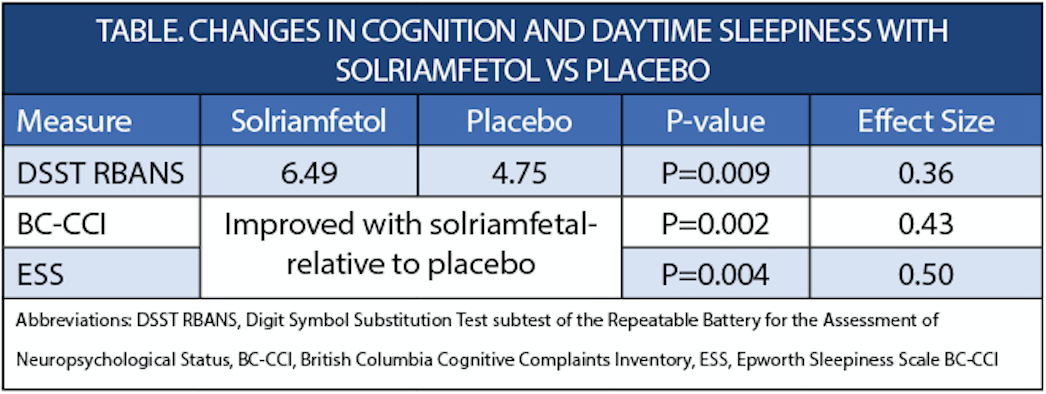
In the SHARP (NCT04789174) study, individuals with excessive daytime sleepiness (EDS) associated with obstructive sleep apnea (OSA) who were treated with solriamfetol (Sunosi; Axsome Therapeutics, New York, NY) had a statistically significant improvement in cognitive function compared with placebo. These improvements were measured by the Digit Symbol Substitution Test subtest of the Repeatable Battery for the Assessment of Neuropsychological Status (DSST RBANS) and the British Columbia Cognitive Complaints Inventory (BC-CCI). Improvements in daytime sleepiness were also observed, as measured with the Epworth Sleepiness Scale (ESS).
“Cognitive impairment in patients with EDS associated with OSA is extremely common and one of the most burdensome symptoms for patients,” said Richard Bogan, MD, FCCP, associate clinical professor at the University of South Carolina School of Medicine, associate clinical professor at Medical University of South Carolina, and principal of Bogan Sleep Consultants. “The results of the SHARP study demonstrate a clinically important improvement in cognitive function with Sunosi treatment in cognitively impaired patients with EDS and OSA. Of importance, the results from the patient-reported outcomes are consistent with the objective measures, meaning that patients themselves were able to perceive improvements in their cognitive performance with Sunosi treatment. The ability to address cognitive symptoms would represent an important advancement in the treatment of EDS associated with OSA.”
The SHARP study was a randomized double-blind placebo-controlled, trial with an enrollment of 59 participants with EDS associated with OSA and impaired cognitive function. All participants were treated with solriamfetol for 2 weeks, with the treatment periods separated by 1 week of down-titration and washout, and then with placebo for 2 weeks.
"We are excited by the demonstrated effect of Sunosi on cognition, using both objective and patient-reported outcomes in the SHARP trial in patients with EDS and OSA experiencing cognitive impairment at baseline," said Herriot Tabuteau, MD, chief executive officer of Axsome. "We plan to discuss these results with the FDA as soon as possible. In addition, we recently presented new data demonstrating that solriamfetol has activity at TAAR1, a previously unrecognized target for this molecule which further supports its exploration in cognition."
The most common adverse events with solriamfetol were anxiety (3.4%) and nausea (6.9%). The study had a 96.7% completion rate.
