Solriamfetol Efficacy and Safety for Treatment of Excessive Daytime Sleepiness in People With Narcolepsy or OSA Is Consistent Across Trials
Solriamfetol (Sunosi; Jazz Pharmaceuticals, Philadelphia, PA) has been approved by the Food and Drug Administration for the treatment of excessive daytime sleepiness in people with narcolepsy or obstructive sleep apnea (OSA) and is pending a scheduling decision from the Drug Enforcement Agency (DEA) in mid 2019.
In pooled analysis of data from the TONES 2 and TONES 3 clinical trials (NCT01681121, NCT02348593, NCT02348606), the efficacy and safety of solriamfetol was consistent (Table).
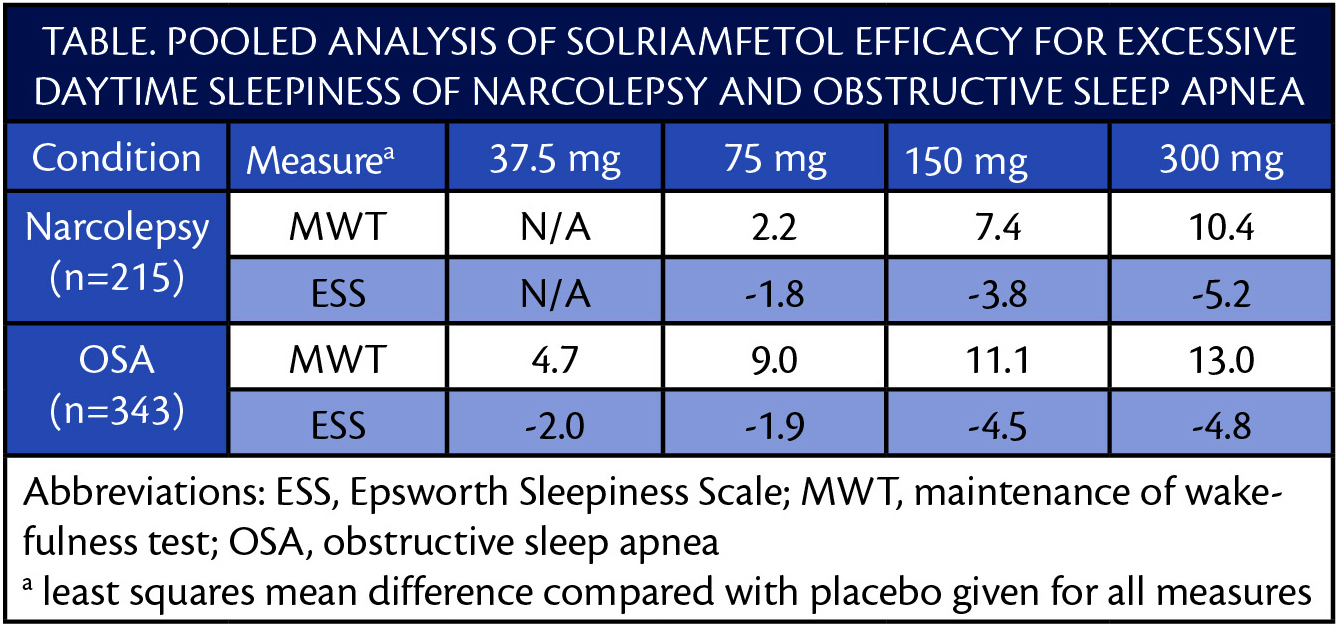
After 12 weeks of treatment, the percentage of participants reported as having improvement on the Patient Global Impression of Change (PGI-C) was increased relative to placebo for both disorders. In individuals with OSA, the efficacy was similar for those who were or were not adherent to treatment of OSA with CPAP.
Jed Black, MD, senior vice president, sleep and CNS Medicine at Jazz Pharmaceuticals and adjunct professor, Stanford University Medical Center, Stanford Center for Sleep Sciences and Medicine, said “What is exciting about Sunosi is that it demonstrates relatively large treatment effects in both the narcolepsy population and the OSA population for the symptom of excessive daytime sleepiness. The effects of excessive daytime sleepiness are profoundly debilitating for these individuals. We encourage clinicians to treat the patient’s excessive daytime sleepiness appropriately and recognize this means optimizing the patient’s primary airway therapy as a first step. Additionally, we are pleased to give clinicians and patients another tool for treating excessive daytime sleepiness.”
The TONES 4 study enrolled participants with OSA who had ESS scores of 10 or more, mean sleep latency less than 30 minutes, and were either currently treated with primary airway therapy, or had attempted use of primary airway therapy for OSA. Further analysis from the study evaluated how participants were titrated to different doses over time and baseline characteristics associated with titration to higher or lower doses. Within the study, participants initiated solriamfetol at a dose of 75 mg and were able to titrate up or down 1 dose level (to 150 mg or a maximum dose of 300 mg) every 3 days after consultation with their clinician over a 2-week period. After titration, participants remained on a stable dose for 2 weeks. Participants receiving 75 mg, 150 mg, or 300 mg of solriamfetol during the stable-dose phase reported improvement 91.3%, 88%, and 75%, respectively.
In open-label studies, participants with narcolepsy (n = 226) or OSA (n = 417) had sustained improvements in quality-of-life (QoL) measures for up to 52 weeks of treatment. Participants mean Functional Outcomes of Sleep Questionnaire short version (FOSQ-10) scores improved by 3.7 points from baseline, and the magnitude of improvement was similar for those with OSA or narcolepsy. Work impairments decreased as measured by the Work Productivity and Activity Impairment Questionnaire: Specific Health Problem scale (WPAI:SHP) by 25% or more. The overall health state of participants also improved as measured by increases in the Short Form Health Survey version 2 (SF-36v2) (mean 3.1 +/- 6.9 for physical component summary score; 4.3 +/- 8.4 for mental component summary score).
The most frequent adverse effects of solriamfetol reported in a long-term, open-label study are headache, nausea, decreased appetite, anxiety, nasopharyngitis, diarrhea, and dry mouth, the incidence of which were similar in those with OSA or narcolepsy.
In a human abuse liability study, the abuse potential of solriamfetol was assessed relative to phentermine, a schedule IV medication. Results from this clinical study demonstrated that solriamfetol (at doses 2, 4, and 8 times the maximum recommended daily dose) produced Drug Liking scores similar to or lower than phentermine.
