Pitolisant Addresses Significant Burdens of Narcolepsy and Need for Treatments With Novel Mechanisms of Action
Coordinated online surveys of individuals with narcolepsy (n = 200) and physicians who treat people with narcolepsy (n = 251) demonstrate significant burden of disease and desire for new effective treatments (94% of both groups) with new mechanisms of action (94.8% of physicians). Average time from symptom onset to diagnosis of narcolepsy was an average of 6.4 years, and misdiagnosis was as high as 54.2%. Although 25.5% of people with narcolepsy reported cataplexy, over half (53.5%) said they knew little or nothing about it.
Among those with narcolepsy, 73.5% said they missed daily activities and 76% said significant life moments were negatively affected by narcolepsy. The percentage of people diagnosed by physicians as having narcolepsy (38.4%) and the percent of people reporting cataplexy (25.5%) were lower than expected based upon existing prevalence data.
Together, these data show a high burden of disease and suggest narcolepsy may be underdiagnosed and, thus, undertreated.
Pitolisant (Harmony Biosciences, Plymouth Meeting, PA), an investigational product, is a first-in-class molecule with a novel mechanism of action that works as a histamine 3 (H3) receptor antagonist/inverse agonist to increase histaminergic transmission in the brain. A new drug application for pitolisant for treatment of excessive daytime sleepiness and cataplexy in adults with narcolepsy was submitted to the Food and Drug Administration (FDA) late last year and is currently under a priority review. In clinical trials, treatment with pitolisant reduced Epsworth Sleepiness Scale (ESS) scores by -5.8 compared with -3.1 for placebo (P = .024) (HARMONY-1 [NCT01067222]) and −5.4 vs −1.9 (P =.0001) (HARMONY-CTP [NCT01800045]). The weekly rate of cataplexy in HARMONY-1 was reduced by 65% with pitolisant vs 9% with placebo (P = .034) and 75% vs 38% in HARMONY-CTP (P < .0001). (Neurology. 2019;92(15S):P3.6-034).
In an open-label phase 3 study, pitolisant was effective as monotherapy in reducing EDS and cataplexy attacks and, when used in combination with stimulant and anticataplexy treatments in patients with residual EDS on entry into the study, demonstrated incremental improvement in wakefulness when added onto other common narcolepsy medications. Pharmacokinetic studies showed no clinically relevant drug-drug interactions between pitolisant and modafinil as well as pitolisant and sodium oxybate.
In the open-label expanded access program, PEACE (NCT03433131), treatment persistance for patients taking pitolisant was high (Figure).
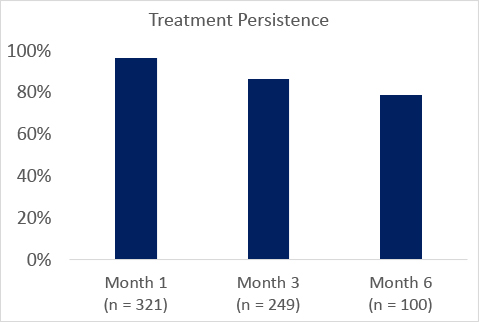
The most commonly reported adverse effects were headache (7.7%), insomnia (4.1%), nausea (4.1%), and anxiety (3.8%) similar to that seen in integrated analysis of the 4 double-blind placebo-controlled randomized clinical trials.
In a human abuse potential study with pitolisant compared to phentermine (C-IV) and placebo, 43 recreational stimulant users were enrolled and 38 completed the study. Pitolisant demonstrated significantly lower drug liking and willingness to take the drug again compared to phentermine (P < .001) and an overall profile similar to placebo. Together with the lack of any signals for abuse potential in clinical trials, these data suggest a low potential for abuse of with pitolisant.
Jeffrey Dayno, MD, chief medical officer of Harmony Biosciences noted, “we have been committed to education regarding the role of histamine in sleep-wake state stability and will continue those efforts. Pitolisant, if approved, would be the first new treatment in over 15 years that treats both excessive daytime sleepiness and cataplexy in adult patients with narcolepsy and we are excited for the opportunity to offer a new treatment option to improve the lives of people living with narcolepsy.”
These data were presented at SLEEP2019, the 33rd annual meeting of the Associated Professional Sleep Societies LLC (APSS), a joint venture of the American Academy of Sleep Medicine and the Sleep Research Society.
