Once-Nightly Sodium Oxybate Improves Narcolepsy Symptoms Regardless of Concomitant Stimulant Use
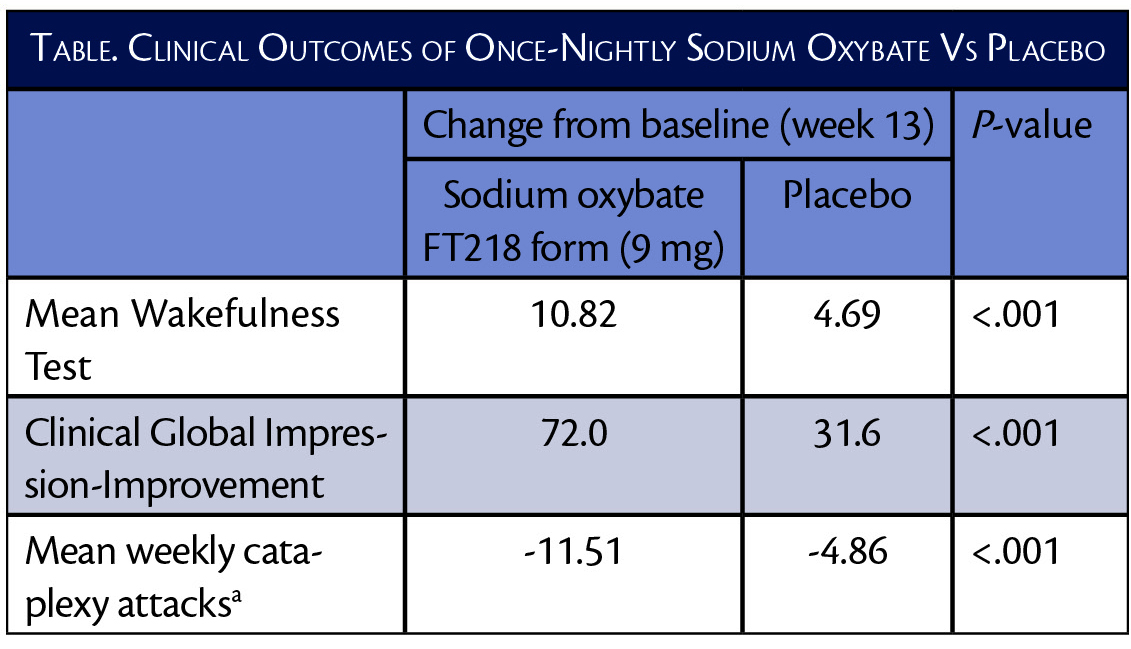
In the REST-ON trial (NCT02720744), treatment with 9 mg of once-nightly sodium oxybate (ON-SXB)(FT218; Avadel Pharmaceuticals, Dublin, Ireland) improved narcolepsy symptoms as measured with the Maintenance of Wakefulness Test (MWT) and Clinical Global Impression-Improvement (CGI-I) score. Those treated with ON-SXB had more than twice as much improvement vs those treated with placebo. The mean number of weekly cataplexy ataxia was also decreased twice as much with the sodium oxybate formulation compared with placebo. This unique formulation of sodium oxybate uses a micropump technology to allow for once nightly dosing, which is not available with existing formulations.
New post hoc analysis now shows that ON-SXB improvement on MWT and CGI occur both with or without concomitant use of stimulants, a common treatment for the symptom of excessive daytime sleepiness (EDS) in people with narcolepsy. Approximately 63% of the safety population (n=212) used stimulants, with modafinil, armodafinil, and dextroamphetamine being used most commonly. At week 3 of treatment, those who had ON-SXB with or without stimulants, respectively, had least-squares mean improvement of 8.32 (95% CI: 2.6-8.1; P<.001) and 7.42 (95% CI: 1.2-7.2; P<.01). In comparison, those treated with placebo with or without stimulants, respectively, had least-squares mean improvement of 3.23 and 2.97 points on the MWT.
On the CGI, approximately 40% of those treated with ON-SXB (with or without stimulants) had much or very much improvement vs 7.7% and 4.4% of those treated with placebo with and without stimulants, respectively. Interestingly, at week 8, the proprotion with improvement on CGI continued growing for all groups, with 66.3% and 54.5% of those treated with ON-SXB with and without stimulants, respectively, having much or very much improvement vs 26.5% and 17.5% with placebo (w/ and w/out stimulants respectively). At 13 weeks, the study endpoint, the proportion having these improvements plateaued for those treated with ON-SXB without stimulants (55.1%) but continued for all other groups (ON-SXB+stimulants: 80.5%; placebo+stimulants: 35.3%; placebo w/out stimulants: 217.2%). At all times, a significantly larger proportion of people treated with ON-SXB with or without stimulants had improvements compared with placebo with or without stimulants (P<.01).
“Avadel is focused on providing a meaningful solution for people with narcolepsy and we are pleased to share further evidence of the clinical benefit of FT218, taken once at bedtime,” said Jennifer Gudeman, PharmD, vice president Medical and Clinical Affairs at Avadel. “We leveraged our unique scientific capabilities to develop a proprietary formulation of sodium oxybate to address the limitations of currently available treatments that require twice-nightly dosing. We believe these new analyses, along with previously presented REST-ON positive Phase 3 data, strengthen the body of evidence demonstrating that FT218, if approved, has the potential to be a transformative treatment for people living with narcolepsy.”
Post hoc analysis also showed that participants treated with ON-SXB had a body mass index (BMI) least-squares mean decrease of -0.5 kg/m2 vs those with placebo, who had a least-squres mean BMI increase of 0.1 kg/m2 from baseline to study end (week 13) (P<.001). Of those treated with ON-SXB, 17.8% had at least a 5% weight loss compared with 3.8% of those who received placebo.
