Once-Nightly Extended Release Formulation of Sodium Oxybate Shows Efficacy for Excessive Daytime Sleepiness and Cataplexy
A phase 3 clinical trial shows that an investigational extended release formulation of sodium oxybate (FT218; Avadel Pharmaceuticals, Dublin, Ireland) increased wakefulness and decreased cataplexy. This unique formulation of sodium oxybate uses a micropump technology to allow for once nightly dosing, which is not available with existing formulations.
In the REST-ON trial (NCT02720744), the highest dose (9 mg) of this formulation of sodium oxybate improved Maintenance of Wakefulness Test (MWT) and Clinical Global Impression-Improvement (CGI-I) scores more than twice as much as placebo. The mean number of weekly cataplexy ataxia was also decreased twice as much with the sodium oxybate formulation compared with placebo.
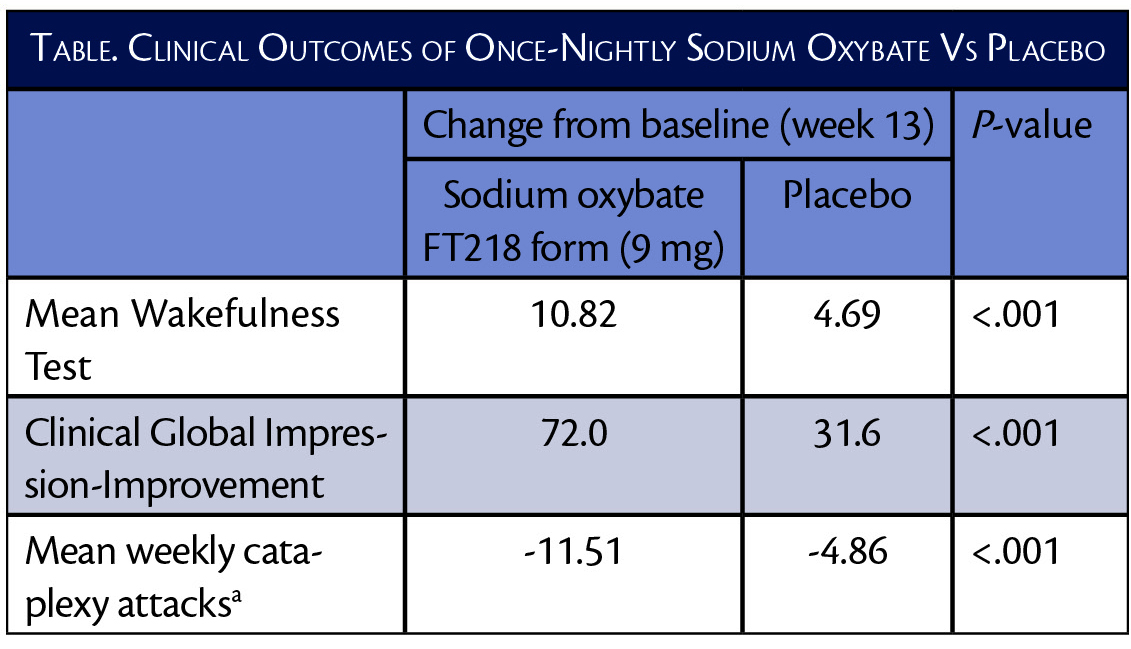
The 7.5 mg and 6 mg doses also demonstrated statistically significant (P<0.001) improvements compared to placebo across the 3 endpoints.
“Once-nightly FT218 delivered a clinically meaningful response within 3 weeks of treatment initiation, which was sustained through each treatment period,” said Jordan Dubow, MD, chief medical officer, Avadel. “Commonly known sodium oxybate adverse reactions occurred at low rates at the highest dose level. We think FT218, if approved, has the potential to be a meaningful contributor to patient care.”
