Long-Term Efficacy and Safety of Low Sodium Oxybate for Idiopathic Hypersomnia
In the double-blind clinical trial (NCT03533114) of lower sodium oxybate (Xywav; Jazz Pharmaceuticals, Philadelphia, PA), individuals with idiopathic hypersomnia (IH) who continued using a titrated stable dose vs those who had it withdrawn maintained symptom improvement. In contrast, those who had low sodium oxybate withdrawn returned to pretitration levels of sleepiness and severity of other IH symptoms.
In long-term extension study of treatment for 24 weeks, symptom reduction was maintained and improvement on Patient Global Impression of Change (PGI-C) occurred for over 90% of participants. Lower levels of absenteeism and presenteeism at work were also observed.
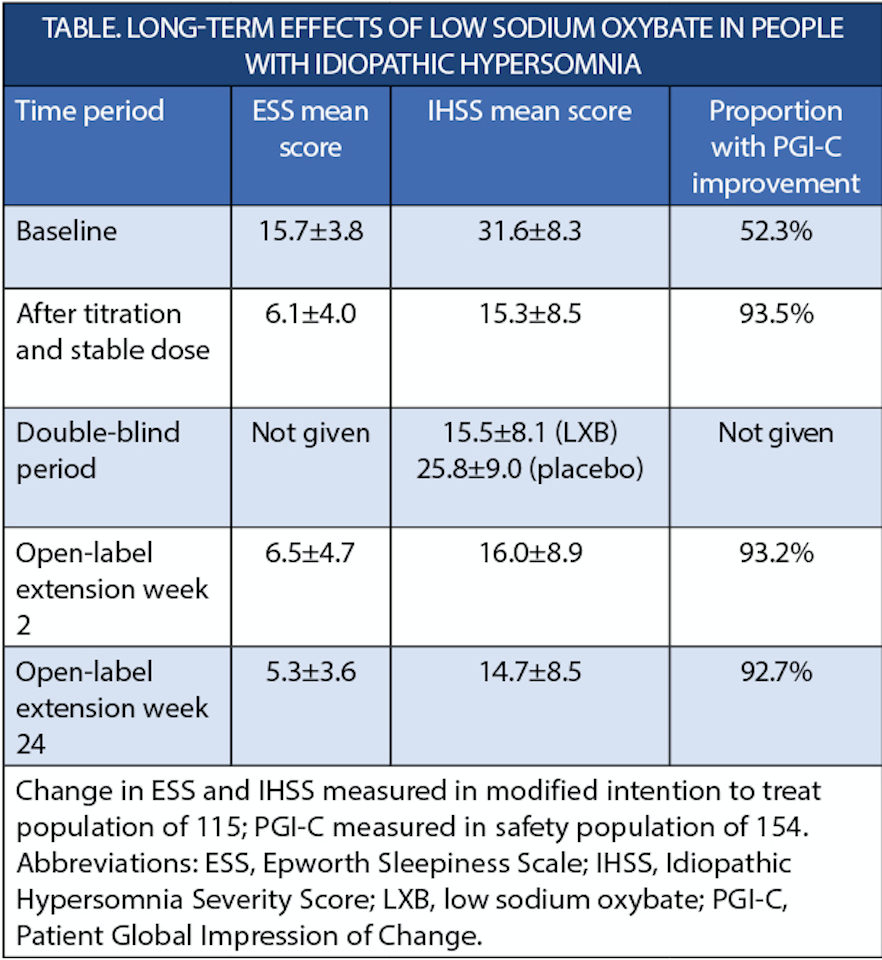
Change in ESS and IHSS measured in modified intention to treat population of 115; PGI-C measured in safety population of 154. Abbreviations: ESS, Epworth Sleepiness Scale; IHSS, Idiopathic Hypersomnia Severity Score; LXB, low sodium oxybate; PGI-C, Patient Global Impression of Change.
Nancy Foldvary-Schaefer, DO, MS, a clinical investigator on the studies and neurologist and director of the Sleep Disorders Center at the Cleveland Clinic, Cleveland OH noted, “It is exciting to finally have an evidence-based treatment for IH approved by the Food and Drug Administration. For many of my patients with IH, we are seeing improvements across multiple domains of life can improve with this new option.”
IH includes not only daytime sleepiness, which also occurs in narcolepsy and other conditions, but also includes sleep inertia, which is difficulty waking (ie, hours to full alertness), and short times to fall asleep at bedtime with a tendency to sleep more deeply and longer than average. Any of these symptoms alone can affect a person’s quality of life, but having all 3 together along with the cognitive complaints those symptoms bring, can be extremely disabling. The IH severity scale (IHSS) measures a composite of all these symptoms.
Participants were allowed to continue other treatments for IH, all of which were off label, and most of which were stimulants or wakefulness promoters. Treatment-emergent adverse events were mild, transient and transient, consisting mostly of nausea, headache, dizziness, anxiety, and vomiting.
