FDA Approves Pitolisant-First-in-Class Nonscheduled Treatment for Excessive Daytime Sleepiness in Adults With Narcolepsy
Approval of pitolisant (Wakix; Harmony Biosciences, Plymouth Meeting, PA) by the Food and Drug Administration (FDA) makes it the first and only approved medication for treatment of excessive daytime sleepiness (EDS) in adults with narcolepsy that is not scheduled as a controlled substance by the Drug Enforcement Administration (DEA). Pitolisant is not a stimulant and has the potential to reduce the burden of care for individuals living with narcolepsy and their health care providers.
As a novel first-in-class agent, pitolisant addresses an unmet need to have new options for treating EDS in narcolepsy that was expressed by 94% of both patients (n = 200) and health-care providers (n = 251) in a survey study. In that same online survey, 73.5% of patients said they missed activities of daily living and 76% said they missed significant life events because of narcolepsy.
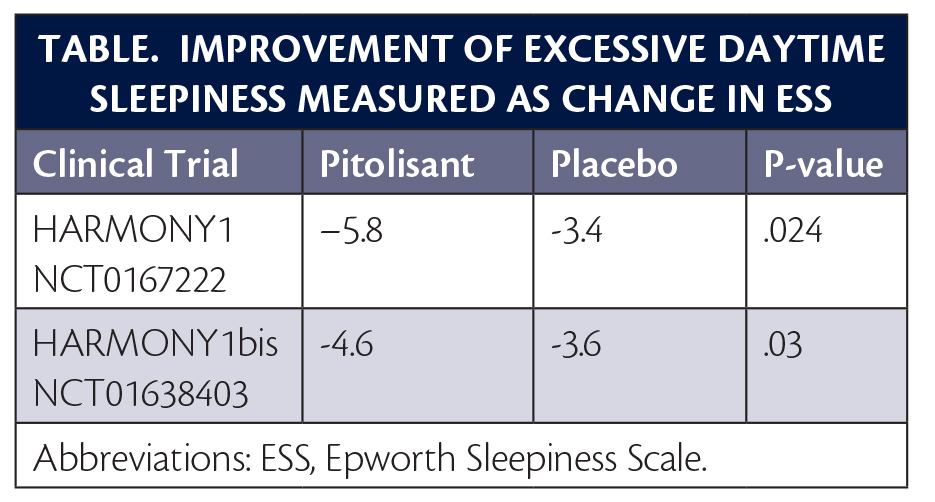
The approval of pitolisant for the treatment of EDS in adults with narcolepsy was based on 2 multicenter randomized double-blind placebo-controlled studies (HARMONY 1 [NCT01067222] and HARMONY 1bis [NCT01638403]). These studies included a total of 261 patients, 75% to 80% of whom had a history of cataplexy. In both of these studies, treatment with pitolisant resulted in statistically significant improvements in EDS as measured by the Epworth Sleepiness Scale (ESS) score (Table). In the participants with narcolepsy (with or without cataplexy) treated with pitolisant, the most common adverse events (occurring in ≥5% of participants and at twice the rate of placebo) were insomnia (6%), nausea (6%), and anxiety (5%).
Jeffrey Dayno, MD chief medical officer of Harmony Biosciences said, “We are excited about the approval of Wakix, a new first-in-class treatment option with a novel histaminergic mechanism of action, which is the first and only approved treatment for adult patients with narcolepsy that is not scheduled as a controlled substance by the DEA. Wakix offers health care providers an important benefit-risk profile to consider in the management of their adult patients with narcolepsy, one which could help address the unmet medical needs of patients living with narcolepsy.”
In the phase 3 open-label, long-term Harmony 3 study (NCT01399606), pitolisant was effective both as monotherapy and in combination with stimulant and anticataplexy treatments in participants with residual EDS being treated with other narcolepsy medications. When used as add-on therapy, pitolisant incrementally improved wakefulness in patients with residual EDS. In a phase 1 drug-drug interaction study, no significant pharmacokinetic interactions were observed when pitolisant was administered with either modafinil or sodium oxybate.
