Effect Size and Number Needed to Treat Suggest Pitolisant Be Considered for First-Line Therapy of Narcolepsy
Post hoc analysis of data from clinical trials of pitolisant (Wakix; Harmony Biosciences, Plymouth Meeting, PA) shows a robust effect size with a low number needed to treat (NNT) (Table). These results suggest pitolisant may be considered for first-line treatment of excessive daytime sleepiness (EDS) and cataplexy in adults with narcolepsy. Pitolisant was approved for the treatment of EDS in 2019 and for cataplexy in 2020.
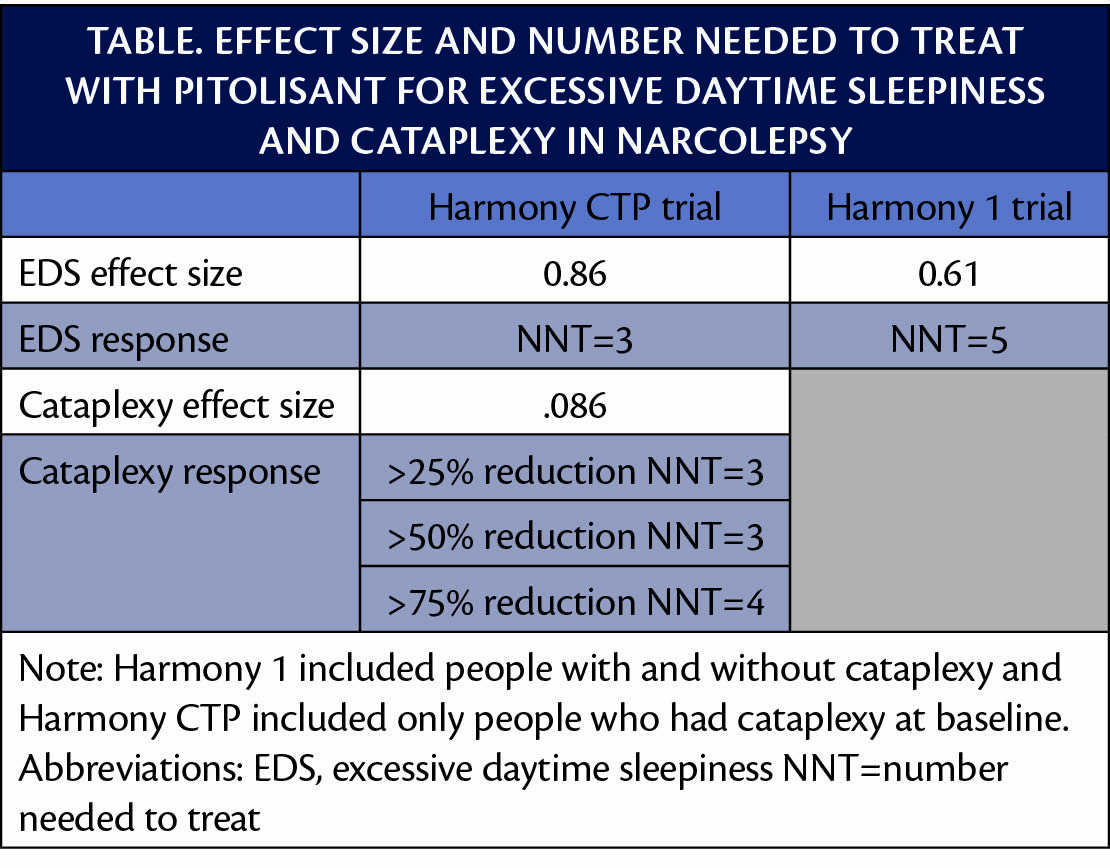
Response to pitolisant for EDS was defined as a reduction on the Epworth Sleepiness Scale (ESS) by at least 3 points or to less than 10 points from a mean baseline of 17.3 to 18.9 across both trials and all treatment groups. For cataplexy, response was defined similarly to that used for other paroxysmal events (eg, seizure or migraine) as decreases of 25%, 50%, and 75% in the number of cataplexy attacks per week, or the weekly rate of cataplexy (WRC). The proportion of participants treated with pitolisant vs placebo who had at least a 25%, 50%, or 75% reduction in WRC was, respectively, 77.8% vs 33.3%, 66.7% vs 25.5%, and 42.6% vs 15.7%.
These data suggest that not only is pitolisant more effective than placebo for treating symptoms of narcolepsy, but also that 1 in 3 people treated with pitolisant for cataplexy can expect to have a 50% to 75% decrease/reduction in these troubling paroxysmal events that interfere with daily functioning. Of 3 to 5 people treated with pitolisant, at least 1 can expect to have improvement in their EDS.
Jeffrey Dayno, MD, chief medical officer of Harmony Biosciences said, “Using NNT and effect size offers a clinically relevant perspective on the efficacy profile of WAKIX and the robust findings of these analyses for both EDS and cataplexy suggests that WAKIX can be considered as first-line therapy for adult patients living with narcolepsy.”
Pitolisant is a first-in-class drug that increases histamine levels in the brain through antagonist/inverse agonist activity at the histamine 3 (H3) receptor.
