Digital Therapeutic for Insomnia Submitted to FDA for Market Authorization as Prescription Treatment
The first prescription digital therapeutic treatment for chronic insomnia (PDT-I) in adults with depression (Somryst; Pear Therapeutics, Boston, MA) has been submitted to the Food and Drug Administration (FDA) for marketing authorization. The PDT -I delivers cognitive behavioral treatment-insomnia (CBT-I)—the evidence-based standard of care for people with insomnia—and a sleep restriction system over 9 weeks that is algorithmically adjusted and personalized over the course of treatment.
Data supporting the application come from 2 clinical trials in which over 1,400 individuals were randomly assigned to receive the PDT-I or an attention-matched digital placebo plus standard care. The placebo controlled for the effect of interacting with a digital intervention with a similar amount of time and attention as required by the PDT-I. As published in the Lancet Psychiatry and shown in the Table below, treatment with PDT-I was effective and safe, improving not only insomnia, but also symptoms of depression, anxiety, and suicidal ideation. No habit-forming effects of treatment or other adverse effects were seen.
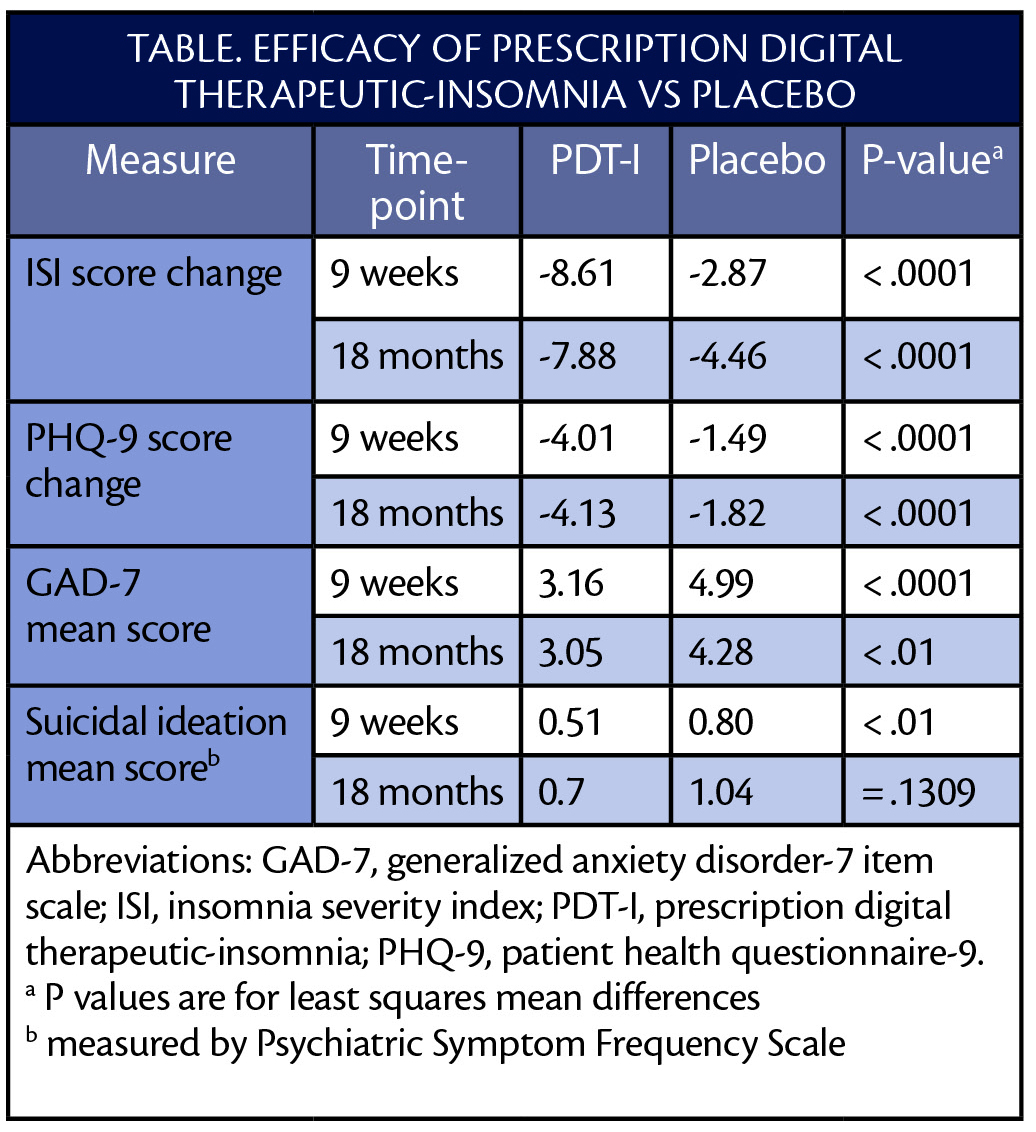
After 9 weeks of treatment, the majority of individuals treated with PDT-I no longer met criteria for diagnosis of insomnia or depression. Of note, efficacy of the PDT-I persisted for at least 18 months.
Despite evidence that CBT-I is highly effective for treatment of insomnia, there is unmet need for treatment because access to therapy is limited both by geographic distribution and total number of people able to provide the therapy. The anytime, anywhere you need it availability of PDT-I could help address this unmet need for many of the 30 million adults with chronic insomnia.
If authorized, this will be the third FDA-approved PDT from Pear Therapeutics. The company has already developed and received FDA approval of PDTs for treatment of addiction disorder and opiate-use disorder (reSet and reSet-O). Pear is 1 of 9 companies invited by the FDA to participate in the FDA’s Software Precertification Pilot Program and underwent the first-ever FDA Excellence Appraisal in May 2019—an onsite evaluation of product quality, patient safety, cybersecurity responsibility, clinical responsibility, and a proactive culture. The PDTs currently available from Pear are prescribed by physicians and delivered by the PearConnect program as a time-limited download onto a patient’s own device running iOS or Android.
“Prescription digital therapeutics are a new treatment class that use software applications to directly treat serious disease anytime, anywhere, while also providing clinicians with real-time data on patient progress,” said Corey McCann, MD, PhD, President and CEO of Pear Therapeutics. “Pear Therapeutics is the leader in this emerging space. We are committed to applying the same degree of rigor to PDTs as for drug development to ensure that physicians prescribing PDTs may do so with the same confidence they have in any other approved therapy.”
