Vutrisiran Efficacy for hATTR Amyloidosis with Polyneuropathy Maintained After 18 Months of Treatment
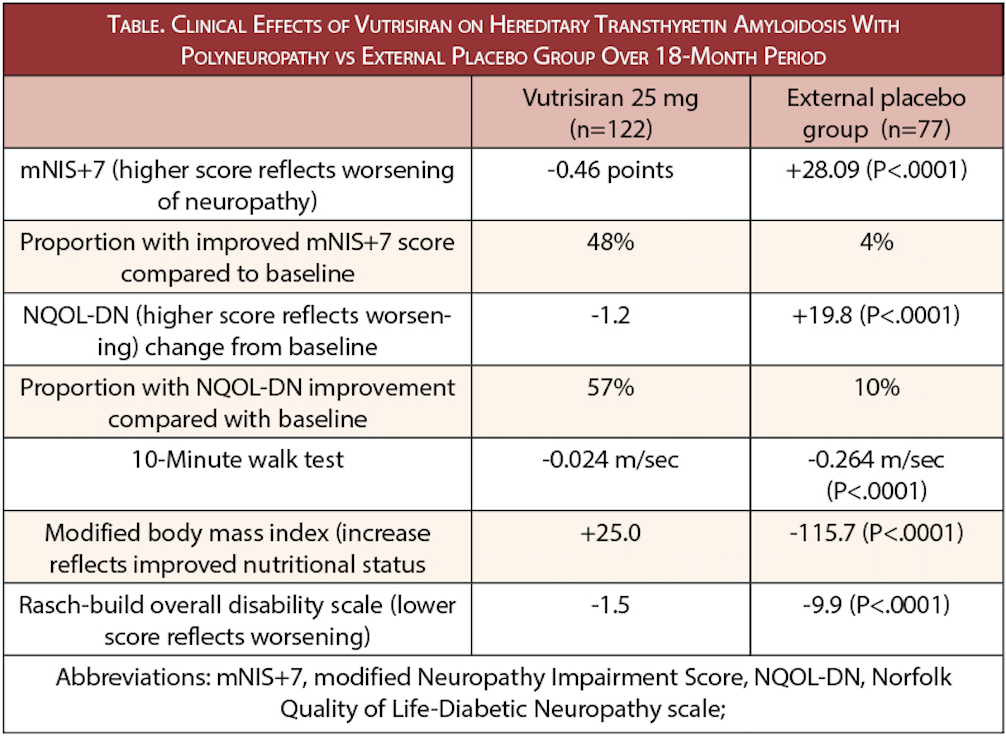
The phase 3 HELIOS-A study (NCT03759379) of vutrisiran (Alnylam Pharmaceuticals, Cambridge, MA) showed efficacy and safety at 9 months and continued treatment through 18 months has shown maintenance of positive results (Table). Noninferiority to the positive comparator patisiran (Alnylam) was demonstrated by an 88% reduction in serum transthyretin levels.
Exploratory analysis also showed potential improvement in cardiac health with vutrisiran treatment. Levels of the cardiac stress biomarker N-terminal-prohormone BNP (NT-proBNP) were lower vs the external placebo group (0.94 vs 1.96). Possible improvement in echocardiograhic parameters and evidence of reduced amyloid burden on the heart were also observed.
“These HELIOS-A results show that the improvement in neuropathy impairment and QoL observed with vutrisiran at 9 months is maintained through Month 18, with the treatment effect increasing over time and an encouraging safety profile,” said Rena N. Denoncourt, vice president, TTR franchise lead, Alnylam. “Further, we are encouraged by the exploratory cardiac endpoint results, particularly new data indicating that 18 months of vutrisiran treatment resulted in reduced technetium uptake in the heart compared to baseline in the majority of patients who were in a planned cohort, suggesting the potential for amyloid regression.”
No new serious treatment-related adverse events occurred from month 9 to month 18. In the first 9 months a case of treatment-related dyslipidemia and a case of treatment-related urinary tract infection were observed. Only 3 study discontinuations have occurred (1 nonfatal heart failure and 2 deaths, both consider nontreatment related). Injection site reactions were mild and transient in the vutrisiran group, all other adverse events reported were similar between groups treated with vutrisiran or placebo.
Both patisiran and vutrisiran are small interfering RNA therapies that reduce serum transthyretin levels. Patisiran is administered as an intravenous infusion, and vutrisiran is administered as a subcutaneous injection.
