Viltolarsen Treatment for Duchenne Muscular Dystrophy Improves Motor Function
In the long-term phase 2 study (NCT04337112), individuals with Duchenne muscular dystrophy (DMD) treated with viltolarsen (Viltepso; NS Pharma, Paramus, NJ) had sustained motor function compared with individuals in a natural history study. These data were published in theJournal of Neuromuscular Diseases.
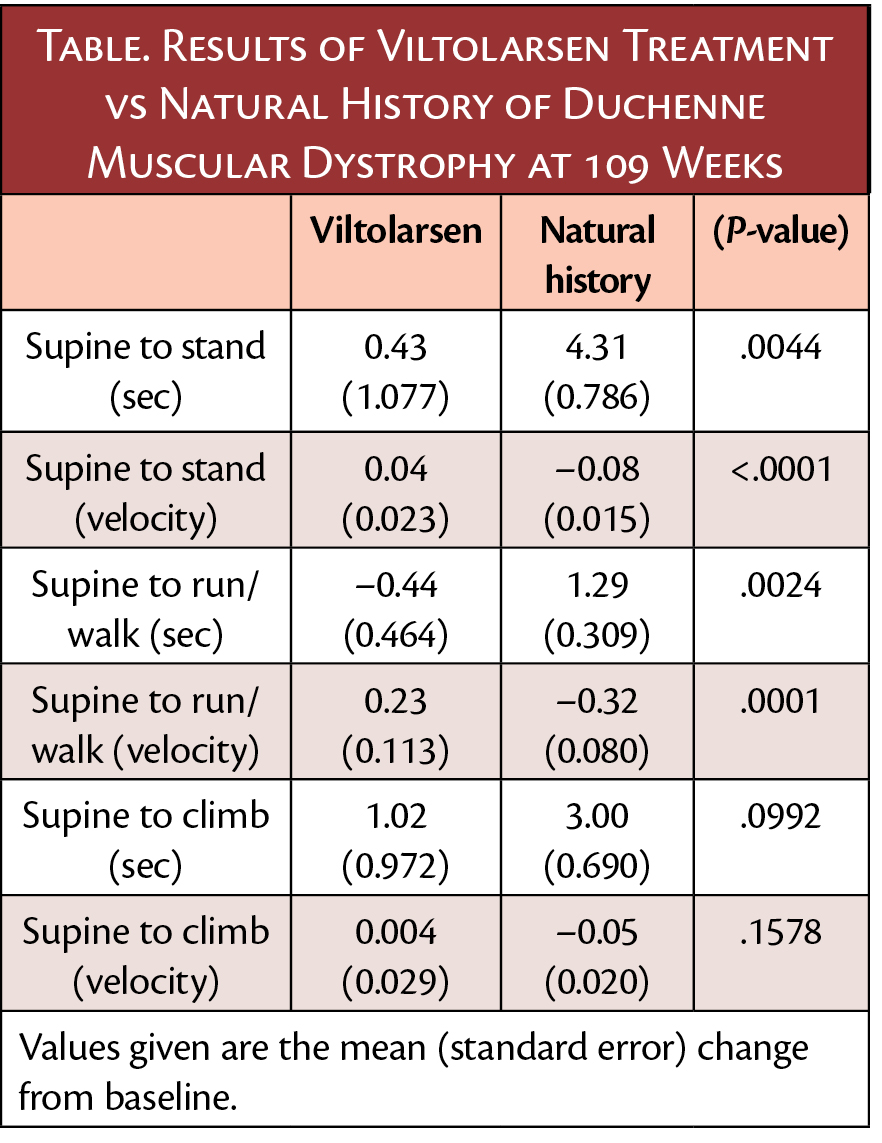
"In this VILTEPSO open-label, long-term extension study, evaluation of functional clinical endpoints demonstrated maintenance of motor function versus functional decline in a historical control group over two years," said Leslie Magnus, MD, vice president, Medical Affairs. "These encouraging interim results with Viltepso support the continued need to research its clinical profile and its potential impact on maintaining mobility."
Participants (n=16) were followed for 109 weeks during which they received open-label treatment with viltolarsen and had assessments at 37, 49, 73 and 109 weeks of timed function tests (Time to Stand, Time to Run/Walk, 6-Minute Walk Test). Results for this cohort were compared to a matched DMD historical control group (Cooperative International Neuromuscular Research Group Duchenne Natural History Study). .
The confirmatory phase 3 RACER53 trial (NCT04768062), evaluating the efficacy of viltolarsen vs placebo for DMD amenable to exon 53 skipping is ongoing.
