Valbenazine Improvements for Tardive Dyskinesia Are Sustained in People Taking Different Neuropsychiatric Drugs
Pooled data analysis from 2 phase 3 trials shows that valbenazine (Ingrezza; Neurocrine Biosciences, San Diego, CA), approved for treatment of tardive dyskinesia (TD) in adults, has sustained efficacy whether or not other neuropsychiatric drugs are taken concomitantly. The efficacy and safety of valbenazine in subgroups was similar to that seen in pivotal trials participants taking antipsychotics (typical or atypical), antidepressants, anxiolytics (benzodiazepine or other), or anticholinergics.
Participants in KINECT 3 (NCT02274558) and KINECT 4 (NCT02405091) were able to continue taking neuropsychiatric drugs needed for psychiatric conditions while receiving once-daily valbenazine (40 or 80 mg) for 48 weeks. The change from baseline in the Abnormal Involuntary Movement Scale (AIMS) total score was measured for all participants and pooled data from both trials was analyzed by subgroups taking different neuropsychiatric drugs. Participants (n = 304) could be in more than 1 group and 85.5% took antipsychotics, 64.5% antidepressants, 30.3% anxiolytics, and 32.2% anticholinergics.
Eiry W. Roberts, MD, chief medical officer of Neurocrine Biosciences noted that, “Ingrezza is a simple to use, once-daily therapy that provides benefit for many patients as early as 2 weeks after initiation. Long-term data now show that benefits of Ingrezza are sustained over many weeks of treatment. People with tardive dyskinesia taking valbenazine can continue treating their underlying psychiatric disorders, from bipolar disorder to schizophrenia to depression, without concern that this will change the efficacy of Ingrezza.”
Significant improvement from baseline AIMS score was seen in all groups (P < .05). Those taking anticholinergic drugs were the only subgroup who had a lower responder rate compared to those who did not. The percentage of participants taking any other neuropsychiatric drug who had more than a 50% total score improvement was 68.9% for those taking antipsychotics vs 53.6% for those who did not; for antidepressants 70.5% vs 57.9%; anxiolytics 72.4% vs 63.6%, and anticholinergics 51.9% vs 72.4%).
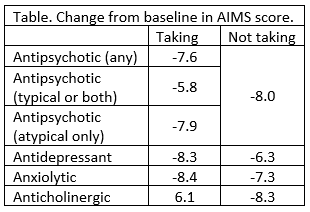
Valbenazine is a novel and highly selective inhibitor of vesicular monoamine transporter 2 (VMAT2). These findings also suggest that valbenazine directly affects the pathophysiologic mechanisms of TD.
