Ublituximab Treatment Lessened Disability for People With Multiple Sclerosis
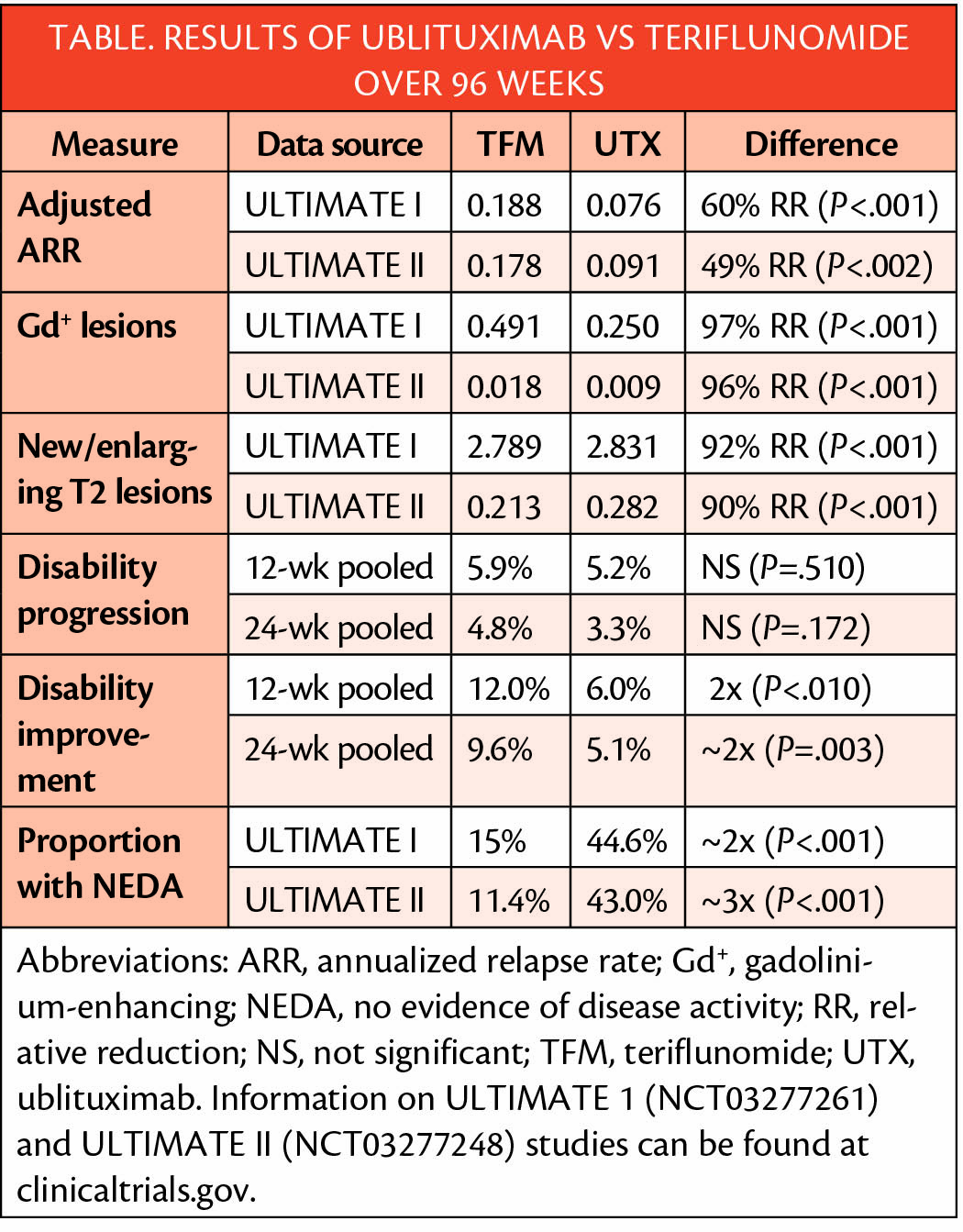
Treatment of people with relapsing multiple sclerosis (MS) with ublituximab (UTX)(TG Therapeutics; New York, NY) slowed progression of MS as measured with biomarkers and disability progression. Compared with teriflunomide (TFM), ublituximab reduced annualized rate of relapse (ARR), gadolinium enhancing (Gd+) lesions, and new or enlarging T2 lesions. UTX also increased the rate of no disease activity (NEDA).
Disability improvement occurred in twice as many people treated with UTX and 2 to 3 times as many people treated with UTX achieved no evidence of disease activity (NEDA). A weakness of this study is that over 97% of participants were white.
Adverse events were balanced across the 2 treatment groups and most commonly were headache (both) and nasopharyngitis (TFM). There were 3 cases of malignancy (endometrial and uterine with UTX and tongue with TFM) and 3 deaths occurred (pneumonia, post-measles encephalitis, and salpingitis, only 1 of which (pneumonia) was considered possibly related to treatment. No progressive multifocal leukoencephalopathy occurred. Infusion reactions (43%) and infections and infestations (4.0%) were more common in those treated with UTX. Most infusion reactions were mild and occurred after the first or second infusion. The rate of infusion reactions decreased over time and the number of infusions.
These data come from the ULTIMATE-I (NCT03277261) and ULTIMATE-II (NCT03277248) multicenter double-blind double-dummy active-controlled phase 3 clinical trials and were presented at the American Academy of Neurology Virtual Annual Meeting May 17-22, 2021. Participants randomly assigned to TFM treatment had daily oral doses plus infusion placebo for 96 weeks and were followed for an additional 20 weeks. Those randomly assigned to receive ublituximab had intravenous infusions of UTX 2 weeks apart and then approximately every 6 months plus a daily oral placebo for 96 weeks with additional 20 weeks of follow-up evaluation.
Participants were adults (1,094) with a diagnosis of relapsing MS using the McDonald 2010 criteria, expanded disability status scale scores of 0 to 5.5 who had at least 2 documented relapses in the prior 2 years or 1 relapse or 1 Gd+ lesion in the prior year. Rates of continuation were high in both studies for both teriflunomide (91.5%, 87.5%) and UTX (87.6% and 92.4%).
UTX is a novel monoclonal antibody targeting a unique epitope on the CD20 antigen designed to increase B-cell lysis through antibody-dependent cellular cytotoxicity that may enhance B-cell depletion and allow lower doses and shorter infusions times compared to other antiCD20s antibodies.
