Ublituximab Reduced Annualized Relapse Rate and Disability Progression in Multiple Sclerosis
In the phase 3 ULTIMATE trials (NCT03277261 and NCT03277248), ublituximab (UTX)(TG Therapeutics, New York, NY) decreased (ARR) and disability progression of relapsing multiple sclerosis (RMS) more than the comparator teriflunomide as detailed in the table.
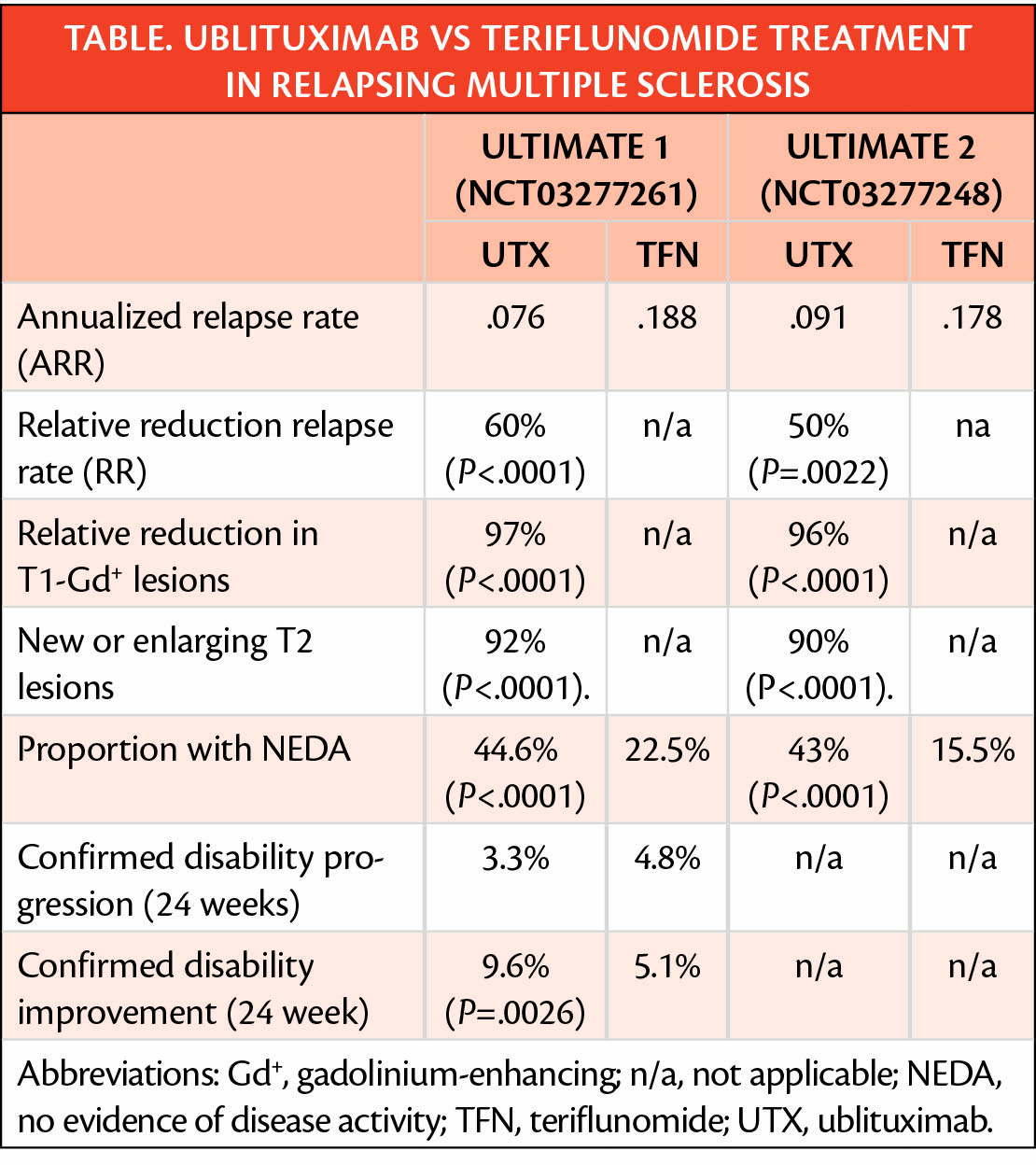
Individuals were randomly assigned 1 to 1 to receive treatment with ublituximab 150 mg in a 4-hour infusion of ublituximab followed by a 1-hour 450 mg infusion every 6 months plus daily oral placebo or teriflunomide 14 mg/day plus placebo infusions. The most common adverse events associated with ublituximab were infusion-related reactions (47.7% treated with ublituximab had at least 1 infusion-related reaction vs 12.2% of those treated placebo infusion).
The studies were conducted under Special Protocol Assessment (SPA) agreement with the Food and Drug Administration (FDA). Data is expected to support a Biologics License Application (BLA) submission to the FDA for use of ublituximab to treat RMS. The results of the study were presented during the 7th Congress of the European Academy of Neurology (EAN).
