Trofinetide Demonstrates Efficacy and Safety for Treating Rett Syndrome
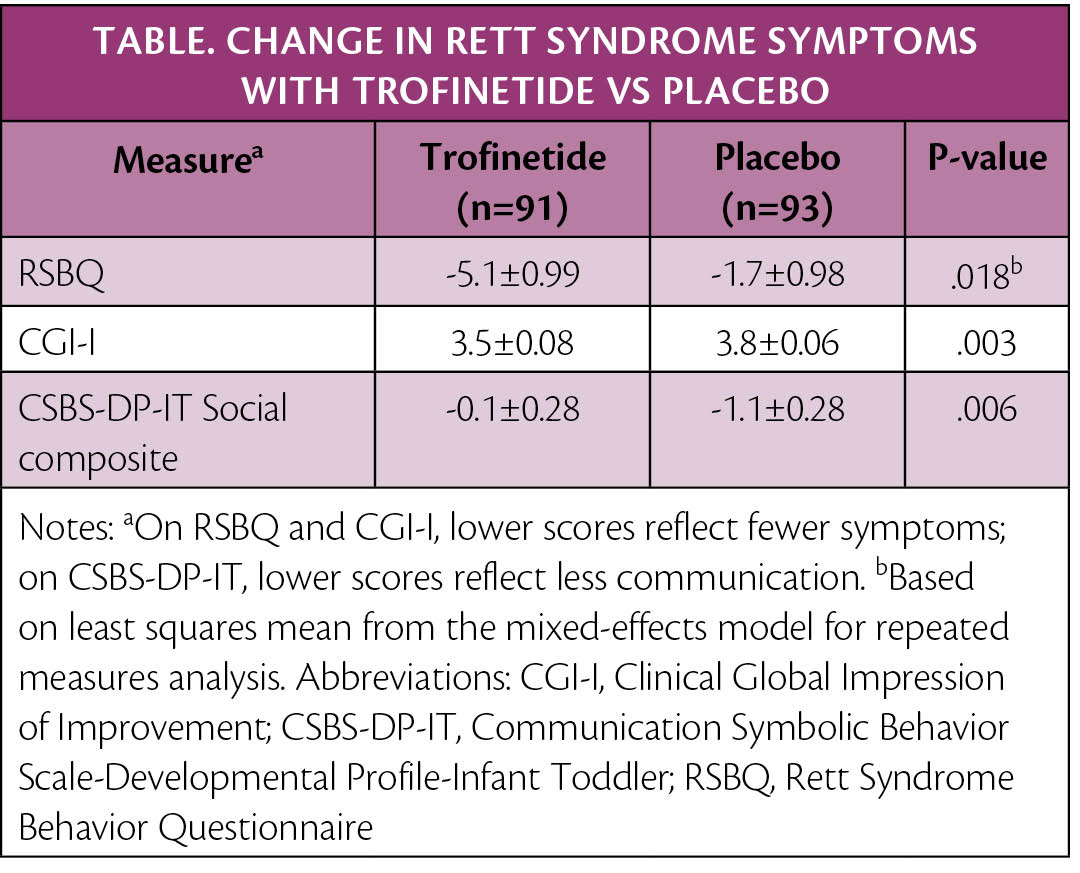
In a phase 3 study (NCT04181723), participants with Rett syndrome who were treated with trofinetide (Acadia Pharmaceuticals, San Diego, CA) vs placebo had statistically significant improvements in the core symptoms of Rett syndrome, including behavior and communication. Improvements on the Rett Syndrome Behavior Questionnaire occurred across all domains, including mood, breathing, hand behavior, repetitive facial movements, body rocking and expressionless face, night-time behavior, fear and anxiety, and walking/standing.
The most common treatment-emergent adverse events were diarrhea, which was mostly mild or moderate, and vomiting. No clinically significant dehydration occurred with diarrhea or vomiting.
Participants in this study were age 5 to 20 years with genetically verified Rett syndrome (ie, a known variant in the methyl-CpG binding protein 2 [MECP2] gene) who had not experienced developmental regression in the 6 months before screening or a change in seizure pattern in the 8 weeks before screening. Inclusion criteria specified participants would have scores of 10 to 36 on the Rett Syndrome Severity Scale at baseline; however, 1 participant had a score of 40.
Trofinetide was administered orally or via a gastrostomy tube (38% of participants). Rett syndrome symptoms were assessed by caregivers using the RSBQ, which is a 90-point scale with higher scores reflecting more impairments, and by clinicians using the Clinical Global Impression-Improvement scale, which is a 7-point scale with Rett syndrome-specific anchors. Communication improvement was measured with the Communication and Symbolic Behavior Scales—Developmental Profile—Infant Toddler, a 13-item questionnaire completed by caregivers.
