Treatment with Eptinezumab Converts Chronic Migraine to Episodic Migraine
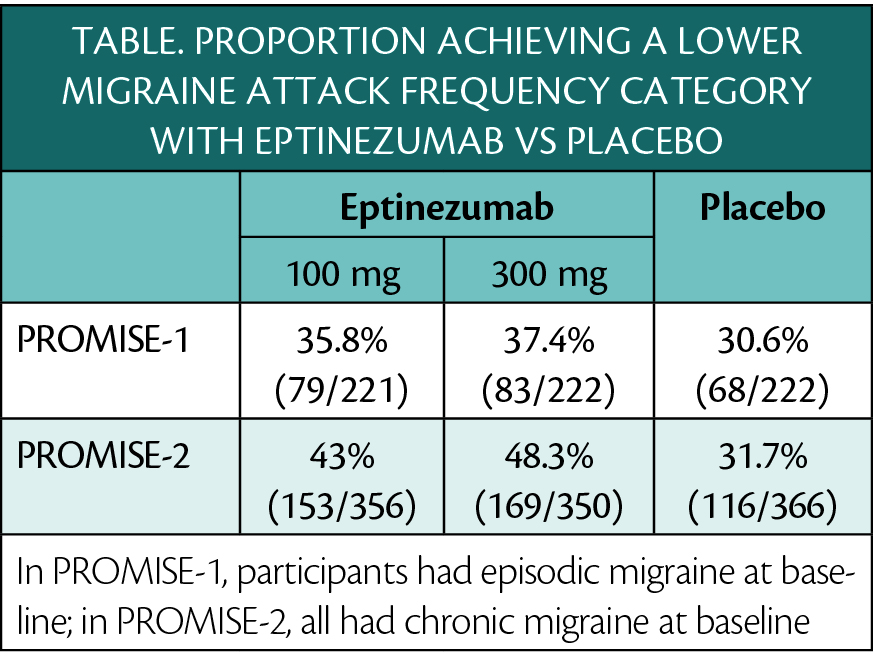
In a post-hoc analysis of the pivotal phase 3 studies, PROMISE-1 (NCT02559895) and PROMISE-2 (NCT02974153), of eptinezumab (Vyepti; Lundbeck, Deerfield, IL), participants treated with eptinezumab for 6 months experienced enough reduction in headache frequency to change their diagnosis from chronic to episodic migraine. In this analysis, chronic migraine was defined as 15 or more monthly headache days (MHD), high-frequency episodic migraine as 10 to 14 MHDs, low-frequency episodic migraine as 4 to 9 MHDs, and very-low-frequency episodic migraine as 3 or fewer MHDs.
“As a physician treating migraine patients every day, it is great to see the growing real-world and clinical evidence supporting the role eptinezumab can play in migraine prevention,” said Paul K. Winner, DO, FAAN, FAHS, Palm Beach Headache Center. “These data contribute to our long-term understanding of VYEPTI and the people who may benefit from this treatment.”
For participants who had a suboptimal response to their first dose (<50% reduction in monthly migraine days [MMD]) many achieved an optimal response with a second dose. Among those with suboptimal response, a second dose response was achieved by 37% (71/192) with eptinezumab vs 33.9% (42/124) with placebo in PROMISE-1 and 28.8% (79/274) vs 18.5% (38/205) in PROMISE-2. The probability of a second-dose response was related to percent change in MMD after the first dose and, in PROMISE-2 only, change on the 6-item
Headache Impact Test (HIT-6) total score.
Additionally, in the phase 3b DELIVER trial (NCT04418765), participants who had experienced 2 to 4 treatment failures with different migraine-preventive medications had greater MMD reductions in MMD with eptinezumab vs placebo for 12 weeks. In episodic migraine, reductions were -3.1 and -4.0 MMD with 100 and 300 mg eptinezumab, respectively, vs -1.0 with placebo. In chronic migraine, MMD decreased 6.5 and 6.6 days with 100 and 400 mg eptinezumab vs 3.3 days with placebo. In medication-overuse headache, mean MMDs decreased 5.6 and 7.3 days with 100 and 300 mg eptinezumab vs 2.3 with placebo.
“These data continue to show the clinical benefit of VYEPTI as a preventive treatment option for people with migraine,” said Marija Geertsen, MD, vice president, Medical Affairs, Lundbeck. “We remain committed to evolving and improving migraine care for those who are highly impacted by migraine and seeking different options to help break the vicious cycle of increasing migraine attacks and more acute treatment use.”
The most common adverse reactions with eptinezumab treatment were nasopharyngitis and hypersensitivity.
