Tolebrutinib Treatment Reduces Disease Activity in Relapsing Multiple Sclerosis
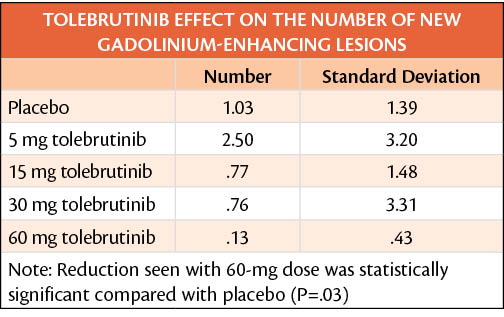
In a phase 2b clinical trial (NCT03889639), tolebrutinib (SAR442168; Sanofi, Bridgewater, NJ) treatment led to a dose-dependent reduction in gadolinium-enhancing (Gd+) lesions in individuals with relapsing multiple sclerosis (MS). The highest dose of 60 mg. A dose-response correlation was seen between tolebrutinib, an inhibitor of Bruton tyrosine kinase (BTK) and reductions in Gd+ lesions.
"There is a growing body of evidence which supports that BTK inhibition may be an appealing, highly effective way to treat relapsing forms of MS. Findings from this study are compelling because they explore the role of microglia in smoldering MS lesions as well as the potential for this specific BTK inhibitor, tolebrutinib, to affect chronic active lesions that are typically associated with disease progression,” said Anthony Traboulsee, MD, professor and research chair, MS Society of Canada at University of British Columbia and phase 2b study investigator. “The results from this study further validate the need to more broadly evaluate the capacity for a CNS penetrating immunomodulator to reduce lesions associated with chronic neuroinflammation and disability progression in patients with MS.”
A serious adverse event was reported in 1 participant in the 60-mg tolebrutinib treatment group, who was admitted to the hospital because of an MS relapse. The most common adverse event during tolebrutinib treatment was headache. No safety-related discontinuations or treatment-related deaths occurred in the study.
The study was a 16-week randomized double-blind placebo-controlled crossover dose-finding trial. There were 130 eligible participants, age 18 to 55 years, diagnosed with relapsing MS (relapsing-remitting or relapsing secondary progressive). The participants had 1 or more of the following: at least 1 relapse within the previous year, at least 2 relapses within the previous 2 years, or at least 1 active Gd+ brain lesion in the last 6 months. Participants were randomized into crossover cohorts for treatment with different tolebrutinib doses and placebo. The first cohort received tolebrutinib for 12 weeks, then matched for 4 weeks; second cohort received 4 weeks of placebo followed by 12 weeks of tolebrutinib. The participants had MRI scans done at screening and every 4 weeks over 16 weeks.
