T-Cell Therapy Targeting Epstein-Barr Virus Provided Sustained Disability Improvement for Progressive MS in Phase 1a Clinical Trial
An Epstein-Barr virus (EBV)-targeting, off-the-shelf allogeneic T-Cell therapy (ATA188; Atara, South San Francisco, CA) was well tolerated and showed signs of efficacy measured as sustained disability improvement (SDI) in participants with progressive multiple sclerosis (PMS). In this Phase 1a 12-month and open-label extension (OLE) study (NCT03283826), dosing occurred for 2 cycles in the initial 12-month portion of the trial and is occurring annually in the open-label extension. The EBV-targeting allogeneic T-cells have been well tolerated by participants at all doses. No fatalities or only mild toxicities have been reported and the safety profile remains consistent with previously reported data. Additionally, the safety profile of ATA188 when administered to patients treated in the open-label extension beyond a year was also excellent.
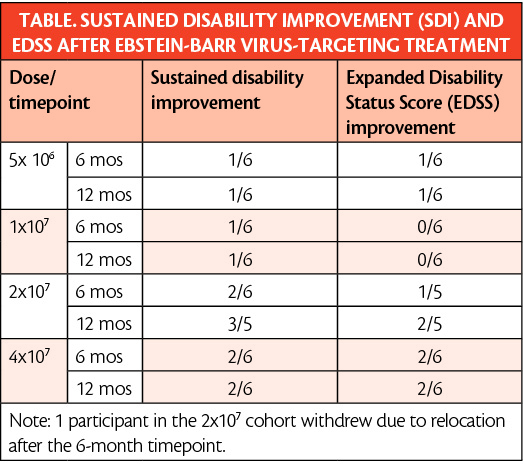
In addition, a larger proportion of participants given higher doses of the T-cell treatment had sustained SDI, suggesting there may be a dose-dependent response to this therapy (Table). Of note, all participants who achieved SDI have maintained SDI over the entire course of the trial to date. Individuals who achieved SDI were also more likely to have improvements in secondary measures including fatigue, the MS impact scale, and the MS walking scale.
Clinical trials with a larger number of participants are needed to confirm these results. A double-blind randomized placebo-controlled phase 1b study (NCT03283826) is actively recruiting participants; for more information about the study and enrollment, contact clinicalstudies@atarabio.com. The study is being amended to increase the sample size and to have SDI as the primary endpoint. Secondary measures include cognition, outpatient ambulatory activity, fatigue, biomarkers, and MRI.
"We are developing a completely novel treatment for MS that targets what we think is a key viral pathogen involved in the pathogenesis of MS. We have shown this treatment is safe and have seen signals of efficacy, said Jakob Dupont, MD, executive vice president and global head, Research and Development at Atara. "These results together with a favorable safety profile highlight the potential of ATA188 for patients with progressive MS and reinforce our excitement around advancing the randomized placebo-controlled portion of the study which is currently enrolling with a recently increased sample size and a switch in the primary endpoint from a biomarker to the clinical measure SDI. We ask the MS community to join us in further study of whether this drug can make a difference for people with progressive MS."
The EBV-targeting T-cells are an off-the-shelf treatment, produced by harvesting T-cell precursors from normal healthy volunteers and coculturing them with cells infected with EBV to produce T-cells that target EBV. Precursors from donors with different HLA profiles are created, allowing the treatment to be personalized for each treated individual's immune profile.
These results were featured in an e-poster at MSVirtual2020: 8th Joint ACTRIMS-ECTRIMS Meeting, held September 11-13, 2020.
