Soticlestat Reduces Seizure Frequency in Developmental and Epileptic Encephalopathies
Long-term treatment with soticlestat (OV935/TAK935; Ovid Therapeutics, New York, NY) in individuals with developmental and epileptic encephalopathies (DEE) reduced seizure frequency in the ENDYMION open-label extension study (NCT03635073). Soticlestat is a selective inhibitor of cholesterol 24-hydroxylase, which has the potential to reduce seizure susceptibility and improve seizure control.
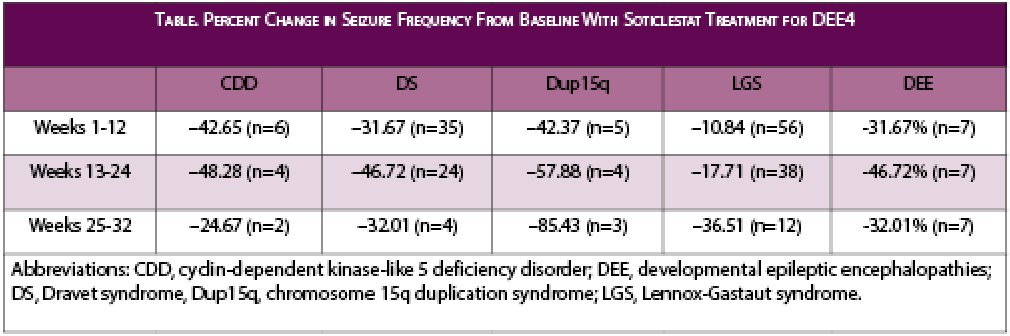
The ENDYMION study is a multicenter open-label extension trial of soticlestat in children and adults with DEEs. Of the 116 eligible participants enrolled, they either completed a previous soticlestat clinical study or received treatment for at least 10 weeks as part of a soticlestat clinical study.
The study included a titration period and then a long-term open-label treatment with soticlestat. Participants who withdrew from the study experienced a 4-week taper and safety follow-up period. Participants received soticlestat twice daily during the treatment period (≤ 600 mg/day, weight-based dosing for participants < 60 kg).
During the study, 83 participants (71.6%) had a treatment-emergent adverse event (TEAE) (mild, 41.4%; moderate, 23.3%; severe, 6.9%). TEAEs occurred in 26.7% (n=31) that were considered related to soticlestat treatment by the investigator. Serious TEAEs occurred in 12 participants (10.3%); none were determined to be related to study treatment. No deaths were reported during the study.
TEAEs that occurred in less than 5% of participants were constipation (5.2), decreased appetite (7.8), diarrhea (5.2), insomnia (5.2), nasopharyngitis (8.6), pyrexia (7.8), seizure, somnolence (7.8), and upper respiratory tract infection (7.8).
