Significant Differences in Therapy Discontinuation Rates Seen in Study of CBRP mAbs and Botox to Treat Migraine
An observational retrospective cohort study using data from the IQVIA PharmMetrics Plus database was used to compare the persistence (the act of continuing a treatment for the entire duration prescribed) of patients with migraine among prescription claims for specific calcitonin gene-related peptide (CGRP) monoclonal antibody (mAb) therapies or Botox (onabotulinumtoxinA; Abbvie, North Chicago, IL). The specific CGRP mAB therapies included in this study were Aimovig (erenumab; Amgen, Thousand Oaks, CA; Novartis, Basel, Switzerland); Ajovy (fremanezumab; Teva Pharmaceuticals USA, Parsippany-Troy Hills, NJ); and Emgality (galcanezumab; Lilly, Indianapolis, IN); and Vyepti (eptinizumab; Lundbeck, Cophenhagen, Denmark). Researchers found that the probability of remaining on therapy after a 15 day gap was similar in those taking either Vyepti or Botox.
Eligible patients included adults (>18 y) with migraine who had at least 1 prescription claim(s) for one of the CGRP mAb treatments studied or Botox, had a minimum of ≥ 12 months of continuous medical and pharmacy coverage, and had 2 or more claims associated with a diagnosis code for migraine ≥ 30 days apart. A 15-day gap in therapy beginning from the date after the last day of therapy as indicated by the days’ supply of the drug was considered as discontinuation. Overall, 66,567 patients with mean ages of 45-47 years with a most recent treatment episode starting after June 25, 2020 were included in the analysis. The study population consisted of primarily females (86%-90%).
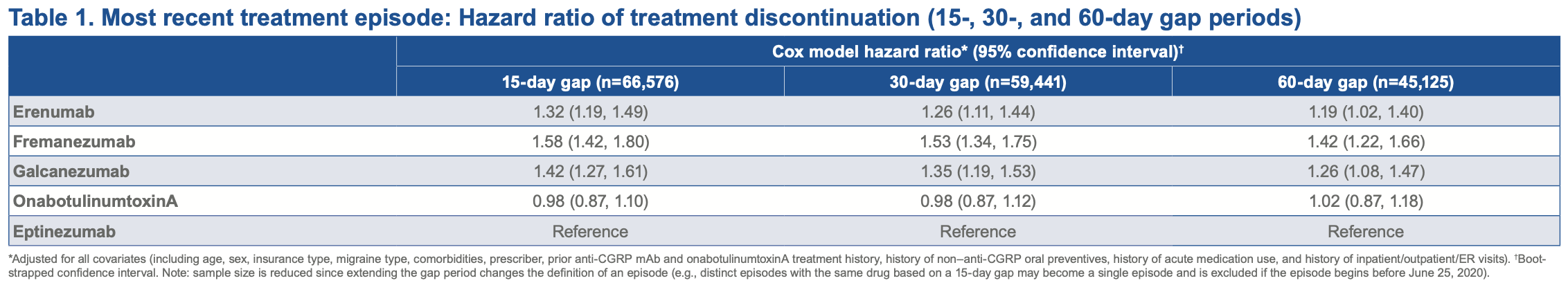
The rate of discontinuation of subcutaneous CGRP mAbs was significantly higher compared with Vyepti (Table 1) based on 15-day, 30-day, and 60-day treatment gaps among patients with migraine with a history of prior CGRP mAb or Botox use. Patients on subcutaneous CGRP mAbs had a 32% (Aimovig), 42% (Emgality), or 58% (Ajovy) higher discontinuation hazard than those receiving Vyepti based on a 15-day treatment gap. The rate of discontinuation with subcutaneous CGRP mAbs in patients who newly initiated an CGRP mAb or Botox was significantly higher compared with Vyepti based on a 15-day treatment gap but not based on 30-day and 60-day gaps. New users of subcutaneous CGRP mAbs had higher discontinuation rates (48% [Aimovig]; 55% [Emgality]; or 68% [Ajovy]) compared with new users of Vyepti.
Authors of this study included Brian Talon, Larry Charleston IV, Stephanie J. Nahas, Christine Sullivan, Seema Soni-Brahmbhatt, Carlton Anderson, Steven Kymes, and Stephane A. Regnier.
