Satralizumab, a Subcutaneously Delivered Monoclonal Antibody, Reduces NMOSD Relapses Over 144-Week Period
The Food and Drug Administration (FDA) accepted a biologics license application (BLA) for satralizumab (Genentech, San Francisco, CA) for the treatment of neuromyelitis optica spectrum disorder (NMOSD). In 2 pivotal trials, satralizumab significantly reduced the risk of relapse both as monotherapy and combination therapy, with robust efficacy sustained for 96 weeks. Satralizumab can be self-administered subcutaneously every 4 weeks. A decision on the BLA is expected from the FDA in 2020.
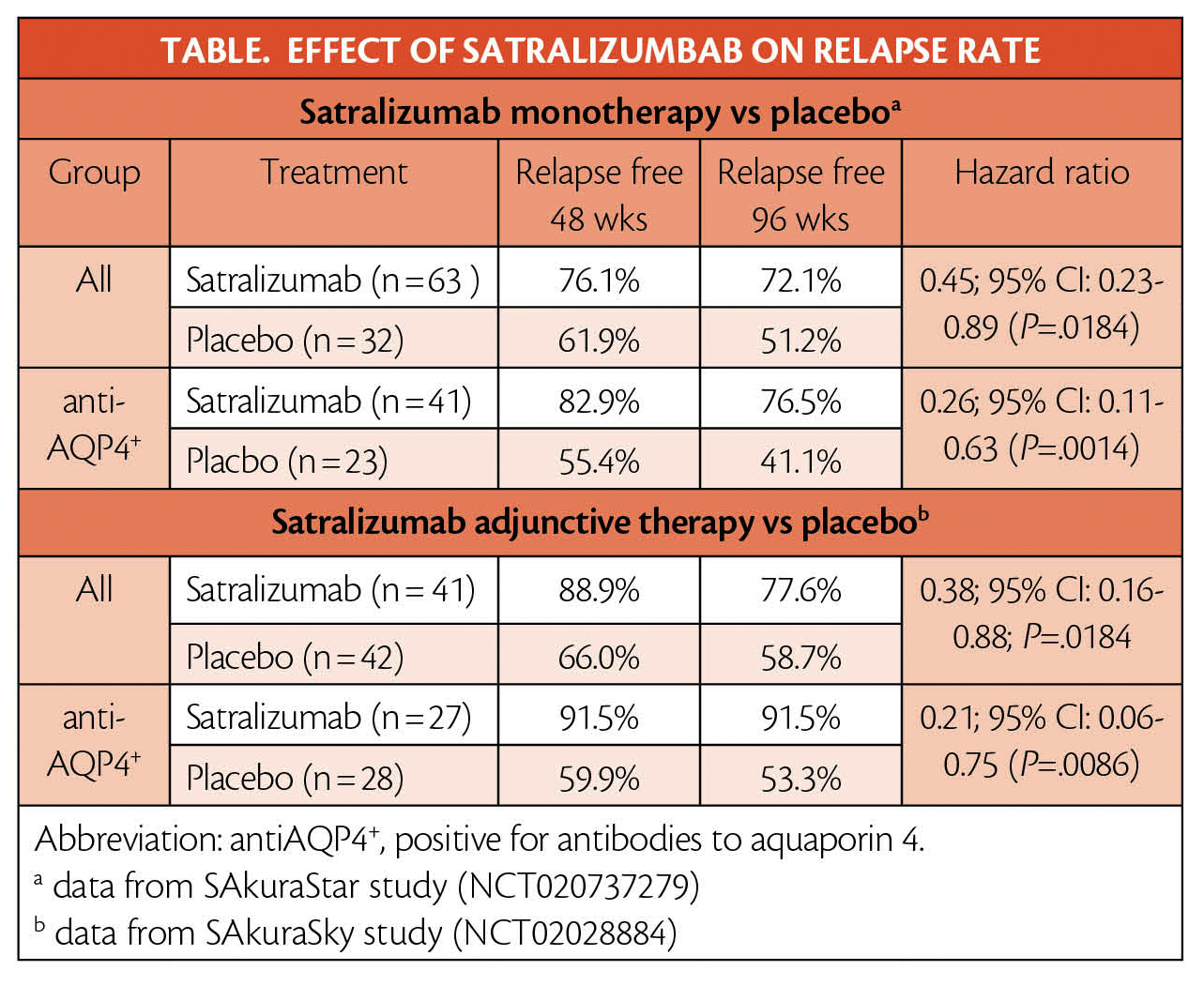
Satralizumab treatment as monotherapy was tested in the SAkuraStar study (NCT020737279) and resulted in a 55% reduction in relapse risk for adults with NMOSD. In the subgroup of people who were positive for antibodies to aquaporin 4 (antiAQP4+), risk of relapse was reduced by 74%. When used in combination with baseline immunosuppressant therapy in the SAkuraSky study (NCT02028884), satralizumab treatment vs placebo provided a 62% relapse risk reduction for adults and adolescents. The subgroup who were antiAQP4+ had risk of relapse reduced by 79% (Table). As reported in the New England Journal of Medicine, the benefit of satralizumab adjunctive therapy vs placebo was sustained through 144 weeks with 74% of people treated with satralizumab remaining relapse free vs 49% with placebo plus immunosuppressants (this increased to 85% vs 53% for the subgroup that is antiAQP4+).
Paris Sidiropoulos, PhD, principal medical science director, Neuroscience at Genentech said, “It’s exciting and encouraging that satralizumab significantly reduced the risk of relapse across a broad range of NMOSD patients, as a monotherapy and in combination with baseline immunosuppressant therapy, and showed robust efficacy sustained for 96 weeks. Notably, SAkuraStar and SAkuraSky represent one of the largest clinical development programs for this rare disease. Satralizumab offers a novel treatment approach through its mechanism of action and subcuteanous administration, so we’re excited to hopefully bring a new option to people with NMOSD in 2020. There is a significant and urgent need for people living with NMOSD to have more approved treatment options with different approaches to treating the disease. Even one NMOSD relapse may lead to blindness, debilitating motor dysfunction, and in some cases death, and until recently, people had no approved treatment options.”
Participants treated with satralizumab had a similar rate of serious adverse events as those treated with placebo. Although infections were the most common adverse events in both trials, more infections occurred with placebo than with satralizumab. In SAkuraSky the most common adverse events were upper respiratory infection, nasopharyngitis, and headache. In SAkuraStar, the most common adverse events were urinary tract infection and upper respiratory tract infection.
Satralizumab is an investigational humanized monoclonal antibody targeting the IL-6 receptor that is thought to be the key driver of the inflammation cascade of NMOSD that leads to damage and disability. If approved, satralizumab will be the first treatment for NMOSD targeting the IL-6 receptor.
