Safety Profiles Similar for Standard and Extended-Interval Dosing of Natalizumab in for Multiple Sclerosis
A study (NCT03689972) was done to assess potential differences in safety and efficacy with different dosing schedules of natalizumab (BG00002; Biogen, Cambridge, MA) for relapsing-remitting multiple sclerosis (MS). The dose schedules evaluated were every 4 weeks as per the approved label and “extended-interval” dosing every 6 weeks, which has been proposed to reduce the risk of progressive multifocal leukoencephalopathy (PML).
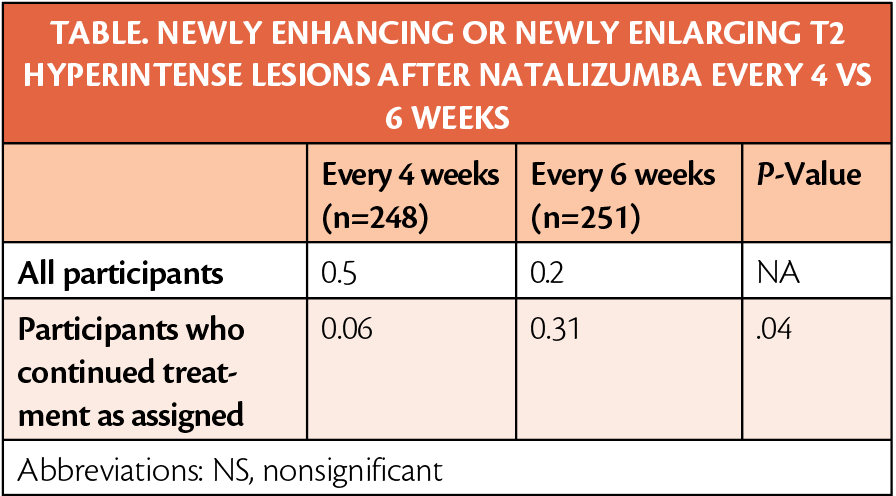
Most lesions found in those treated every 6 weeks occurred in 2 participants, who had 25 or more new lesions. Asymptomatic PML was detected in 1 participant in the 6-week group, who had increased disability but remained asymptomatic 6 months after diagnosis. The rates of serious (7%) and mild-to-moderate adverse events (77%-78%) were similar across the 2 groups.
Although there were fewer lesions with more frequent treatment, the statistical significance of this is unclear because of 2 individuals having a much higher number of lesion burden after treatment. Safety profiles were also similar, but the presence of asymptomatic PML emphasizes the importance of assessing JC viral status and monitoring for PML during treatment with natalizumab.
