Rozanolixizumab Significantly Improves Generalized Myasthenia Gravis
In the phase 3 MycarinG study (NCT03971422), individuals with generalized myasthenia gravis (gMG) treated with rozanolixizumab (UCB7665; UCB, Atlanta, GA) had improvements on the Myasthenia Gravis-Activities of Daily Living (MG-ADL) score. Participants included both individuals with gMG and antibodies to muscle specific kinase (MuSK-Ab+) or gMG and antibodies to the acetylcholine receptor (antiAChR+).
Rozanolixizumab demonstrated statistically significant and clinically meaningful improvements in MG-specific outcomes in patients with MuSK-Ab+ gMG, that were consistent with results in acetylcholine receptor antibody positive (AChR-Ab+) gMG, and the overall population.
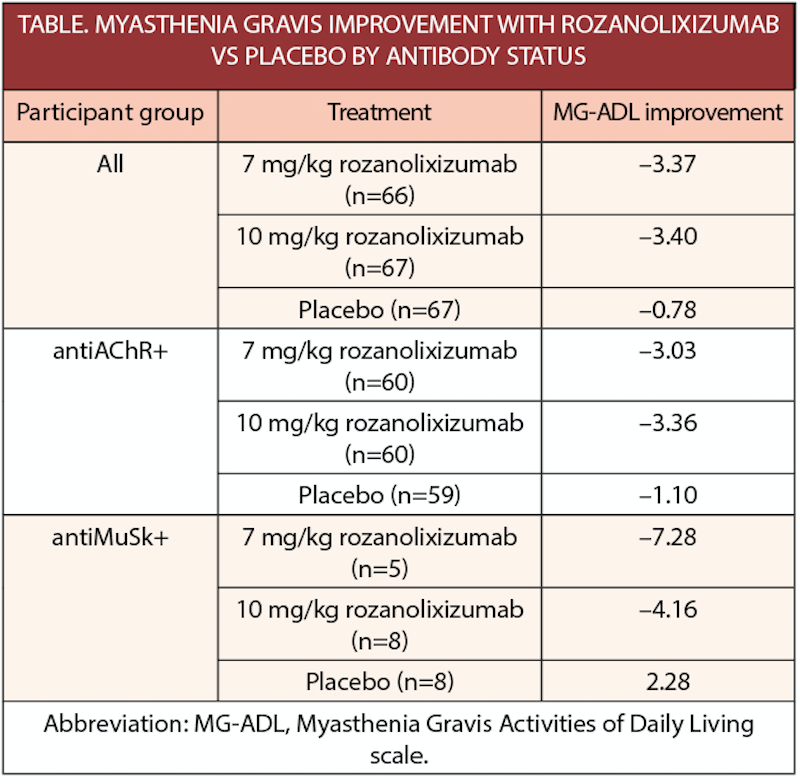
Higher proportions of participants treated with the rozanolixizumab vs placebo achieved a 2.0-point or greater improvement in MG-ADL and a 3.0-point or more improvement in Quantitative Myasthenia Gravis (QMG) scores and Myasthenia Gravis Composite (MGC) scores.
“Patients living with gMG experience high disease and treatment burden resulting in a significant impact on their daily lives. The data being presented at AANEM and the MG Scientific Session reinforce the potential of UCB’s 2 investigational medicines with different mechanisms of action to provide targeted treatment options to patients,” said Iris Loew-Friedrich, executive vice president and chief medical officer at UCB. “We are committed to meeting patients’ needs, regardless of antibody profile, including those with MuSK Ab+ gMG who have particularly limited treatment options. With our gMG pipeline, we hope to address both drivers of disease pathology and which account for approximately 95% of patients living with gMG.”
Rozanolixizumab was well tolerated with most of the treatment emergent adverse events (TEAEs) being mild to moderate in severity. The most frequently reported TEAEs were headache, diarrhea, pyrexia and nausea. A higher proportion of TEAEs occurred with rozanolixizumab vs placebo (81.3% for 7 mg/kg, 82.6% for 10 mg/kg and 67.2% for placebo) and were comparable.
