Rimegepant Reduced Migraine Frequency in Clinical Trial
The rate of monthly migraines was reduced with rimegepant (Nurtec; Biohaven, New Haven, CT) treatment in a randomized placebo-controlled pivotal clinical trial (NCT03732638). The trial evaluated the efficacy and safety of oral rimegepant 75 mg for the preventive treatment of migraine in individuals with episodic or chronic migraine. The study demonstrated a statistically significant reduction from baseline in monthly migraine days in individuals treated with rimegepant compared with placebo.
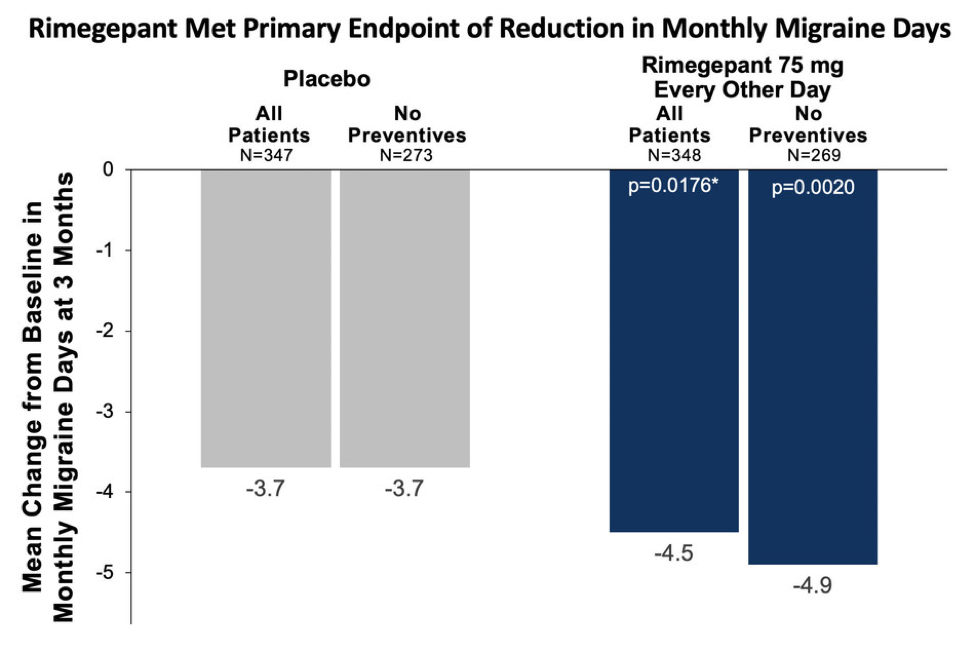
Those receiving rimegepant 75 mg every other day (n=348) experienced a statistically significant 4.5-day reduction from baseline in monthly migraine days, compared with a 3.7-day reduction in the placebo group (n=347; p=.0176). Among study participants who took rimegepant without concomitant preventive treatment (n=273), there was a 4.9-day reduction in monthly migraine days vs a 3.7 day reduction those treated with placebo who were not taking concomitant preventive medication (n=269; nominal p=.0020) (Figure).
A total of 22% of participants were taking a concurrent preventive treatment, including topiramate and amitriptyline. Importantly, 48% those treated with rimegepant had at least a 50% reduction from baseline in the mean number of moderate-to-severe migraine days per month compared with 41% of those treated with placebo.
Richard B. Lipton, MD, professor and vice chair of Neurology at the Albert Einstein College of Medicine and Montefiore Health System, director of the Montefiore Headache Center commented, "I see many patients who are discouraged by the limited current preventive treatment options and continue to look for a better way to prevent disabling migraine attacks. This is the first time that patients may be able to use a single drug for both acute and preventive treatment. Particularly impressive is the fact that the primary outcome measure of reduction in monthly migraine days was achieved with every other day dosing. I believe that rimegepant will fulfill significant unmet needs for my patients as an oral agent that provides acute and preventive treatment benefits."
This pivotal study enrolled participants with both episodic and chronic migraine. The study evaluated the efficacy and safety of rimegepant 75 mg (n=370) dosed every other day for the preventive treatment of migraine vs placebo (n=371) in participants who had migraine for at least 1 year and 4 to 18 moderate to severe migraine attacks per month over 3 months prior to enrollment. During the 1 month observation period, participants experienced on average 10.7 migraine days during the 4 week month, with 7.4 migraine days of moderate to severe pain intensity migraine during the same period.
Robert Croop, MD, chief development officer, Neurology at Biohaven added, "These data demonstrate rimegepant's broad range of clinical activity to potentially provide a new oral preventive treatment option for people with migraine. The magnitude of effect in the rimegepant treated arm with favorable safety and tolerability suggest that rimegepant could be a best-in class oral therapy for both preventive and acute treatment of migraine. We look forward to sharing detailed results from the study at upcoming medical conferences and continuing to advance this innovative oral treatment for the preventive treatment of migraine."
