Propensity Scores Suggest Cladribine More Effective Than Other Disease-Modifying Treatments
Individuals with relapsing multiple sclerosis (RMS) who were treated with cladribine (Mavenclad; EMD Serono, Rockland, MA) had lower annualized relapse rates (ARR) and longer time to first relapse compared with other disease-modifying treatments (DMTs).
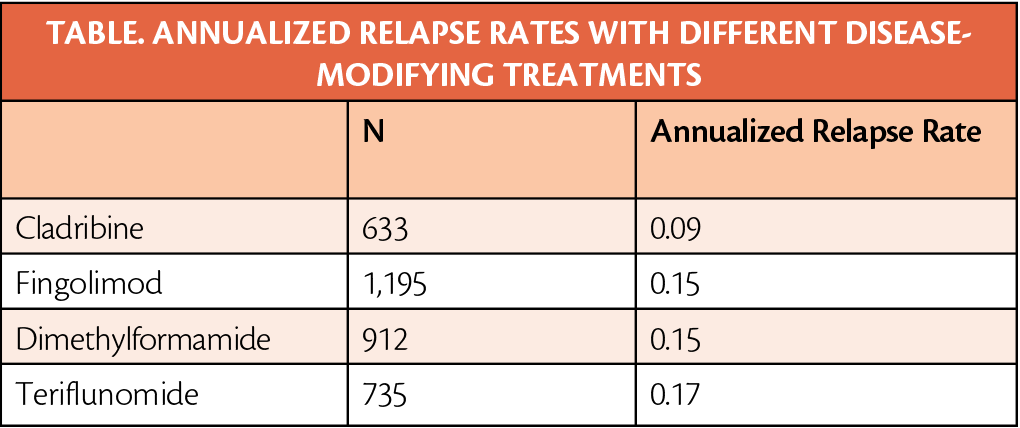
Time to first relapse with cladribine was 40%, 42%, and 67% longer than with fingolimod, dimethylformamide (DMF), and teriflunomide, respectively. Those who were treated with cladribine compared with the other DMTs had a 4 to 6.5 times longer period before switching DMTs.
For individuals with clinically isolated syndrome (CIS), treatment with cladribine resulted in a lower rate of conversion to clinically definite multiple sclerosis (CDMS). There was also a lower risk of relapse (53.2% vs 28.2% with relapse freedom) in those treated with cladribine compared with those who did not receive cladribine.
“It is important in a lifelong disease like MS to continue assessing the efficacy and safety of available treatment options in the real world,” said Helmut Butzkueven, MBBS, FRACP, PhD, Department of Neuroscience, Central Clinical School, Monash University, Melbourne. “This is where the MSBase Registry, using standardized data records from over 79,000 people with MS around the world, can provide information that is not possible to obtain in a randomized clinical trial. This information showed us that in GLIMPSE, Mavenclad had better relapse outcomes and longer treatment persistence compared to other oral DMTs, including fingolimod.”
The data comparing cladribine to other DMTs are from the GLIMPSE study (no NCT number available) and the MSBase registry. Data regarding time to first relapse in people with CIS who were or were not exposed to cladribine are from the exploratory phase 4 CLASSIC-MS study (NCT03961204) following 227 indidivuals who participated in a phase 3 study of cladribine. These data were presented at the Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) meeting Febrary 24-26, 2022.
