Precision Olfactory Drug Delivery to Upper Nasal Cavity With Improved Bioavailablity in Development for Headache, Parkinson’s, and More
Impel Neuropharma (Seattle, WA) is developing new therapeutic formulations for delivery of therapeutic compounds to the upper nasal cavity with their precision olfactory delivery (POD) device. The POD uses a propellant to deliver drugs to the upper nasal cavity, a new route of administration, which can provide higher bioavailability and rapid uptake vs traditional nasal sprays
The POD device is easy to use, requiring only that the nasal tip of the device is inserted into the nostril and squeezed once to activate dose delivery. Coordination of breathing with activation is not required, and it is possible for caregivers to deliver a dose for someone when needed.
A formulation of dihydroergotamine (DHE) for use with the POD device (INP104; Impel Neuropharma) has been developed. In healthy volunteers (n = 27), 30 minutes after INP104 administration, at Cmaxfor INP104, plasma levels of DHE were comparable to that seen 30 minutes after administration of intravenously delivered DHE (IV-DHE) and remained so for 24 hours. At Cmaxfor INP104, average plasma concentration of DHE was 3 to 4 times that after administration of DHE with traditional nasal spray (Migranal; Valeant Pharmaceuticals, Bridgewater, NJ).
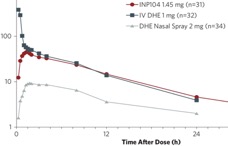
Fewer treatment-emergent adverse events (TEAEs), mostly mild, were seen with INP104 vs IV-DHE; rates of TEAEs were similar between INP104 and DHE delivered via nasal spray. Based on these data, an investigational new drug (IND) application was filed with and accepted by the Food and Drug Administration (FDA). An open-label phase 3 safety study of intermittent use in individuals with migraine has begun. It is expected that an NDA for INP104 will be submitted in the first half of 2020, and if approved, commercial launch in early 2021.
Formulations of levodopa for treatment of Parkinson’s disease (PD) have also been developed for use with the POD device and tested in rats and nonhuman primates. These studies have led to identification of candidates that achieve therapeutic concentrations of levodopa quickly. Lead candidate INP103 is under investigation in a phase 2a trials (NCT03541356).
Formulations of levodopa/carbidopa and olanzapine are among other therapeutic compounds also being developed for use with the POD device.
Jon Congleton, chief executive officer of Impel said, “We are excited to have the opportunity to address unmet medical needs by developing an easy-to-use, highly effective treatment for the 36 million people living with migraine in the US. Impel has tapped into innovation to create transformative therapies by taking proven compounds and delivering them through a new route of administration which could transform the treatment landscape for CNS disorders.
Data from pharmacokinetic study for INP104 and preclinical studies for levodopa formulations were presented at the American Academy of Neurology Annual Meeting May 4-10 in Philadelphia, PA.
