Potassium Channel Modulator Reduces Focal Onset Seizure Frequency in Clinical Trials
In the phase 2b X-TOLE trial, participants ( treated with the positive allosteric modulator of the KCNQ2/3 (Kv7.2/7.3) potassium channel (XEN1101; Xenon Pharmaceuticals, Burnaby, British Columbia) had significant reduction in the frequency of focal onset seizures. Seizure reductions occurred as early as 1 week after beginning treatment.
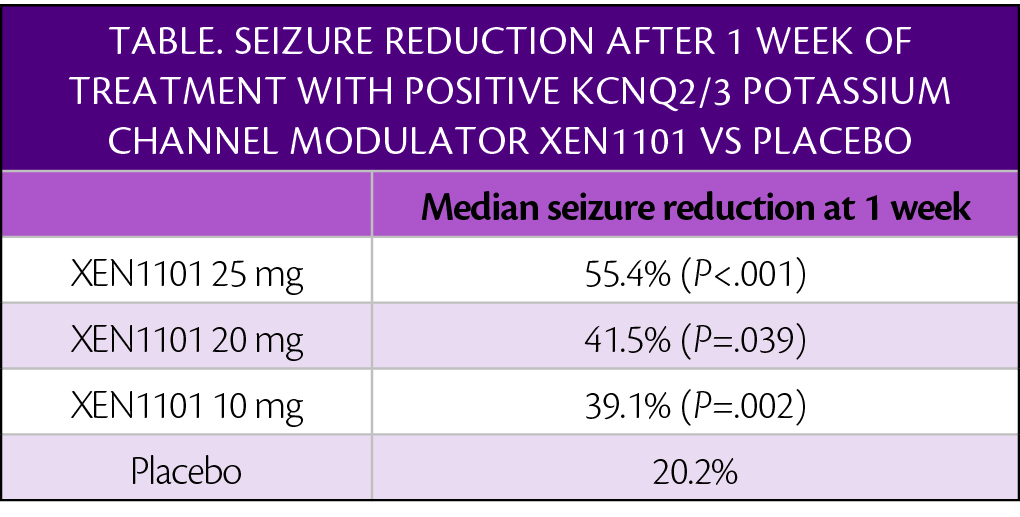
Seizure frequency reductions continued throughout the 8-week double-blind portion of the study and in the first month of the open-label extension (OLE), in which 96% of participants enrolled. Participants remaining in the OLE for at least 3 months had more than a 70% reduction in seizure frequency compared with the baseline measured in the double-blind period. Seizure freedom for at least 6 and 12 months occurred in 19.6% and 9.5% of individuals who entered the OLE.
“Based on these phase 2b efficacy data, we are including the secondary endpoint of ‘week 1 median percent change in seizure frequency’ within the statistical hierarchy of the phase 3 focal onset seizure trials to build upon the differentiated profile of XEN1101,” said Christopher Kenney, MD, FAAN, chief medical officer, Xenon. “Additionally, within our analysis of the open label extension (OLE) population, we are seeing seizure frequency continuing to improve after the double-blind period with patients experiencing increased periods of seizure freedom. At the request of study investigators and based on the potential to continue to provide significant benefit to patients, we are extending the X-TOLE OLE from 3 to 5 years.”
Treatment with this potassium channel modulator was generally well-tolerated with adverse events (AEs) consistent with other antiseizure medicines There were no treatment emergent AEs of pigmentary abnormalities. Urinary retention occurred in 2 participants in the double-blind period and the OLE and was possibly related to study drug; both continued in the study without requiring intervention. Weight changes in the OLE were 1.4 ± 4.5 kg at the 6-month visit and 0.9 ± 6.2 kg at the 12-month visit.
