Stem-Cell Treatment Improves Multiple Sclerosis Disability in Phase 2 Trial
In a phase 2 trial (NCT03799718), 3 repeated administrations of autologous neurotrophic factor-secreting mesenchymal stem cells (MSC-NTF) (NurOwn; BrainStorm Cell Therapeutics, New York, NY) treatment showed improvement for progressive multiple sclerosis (MS) (Table).
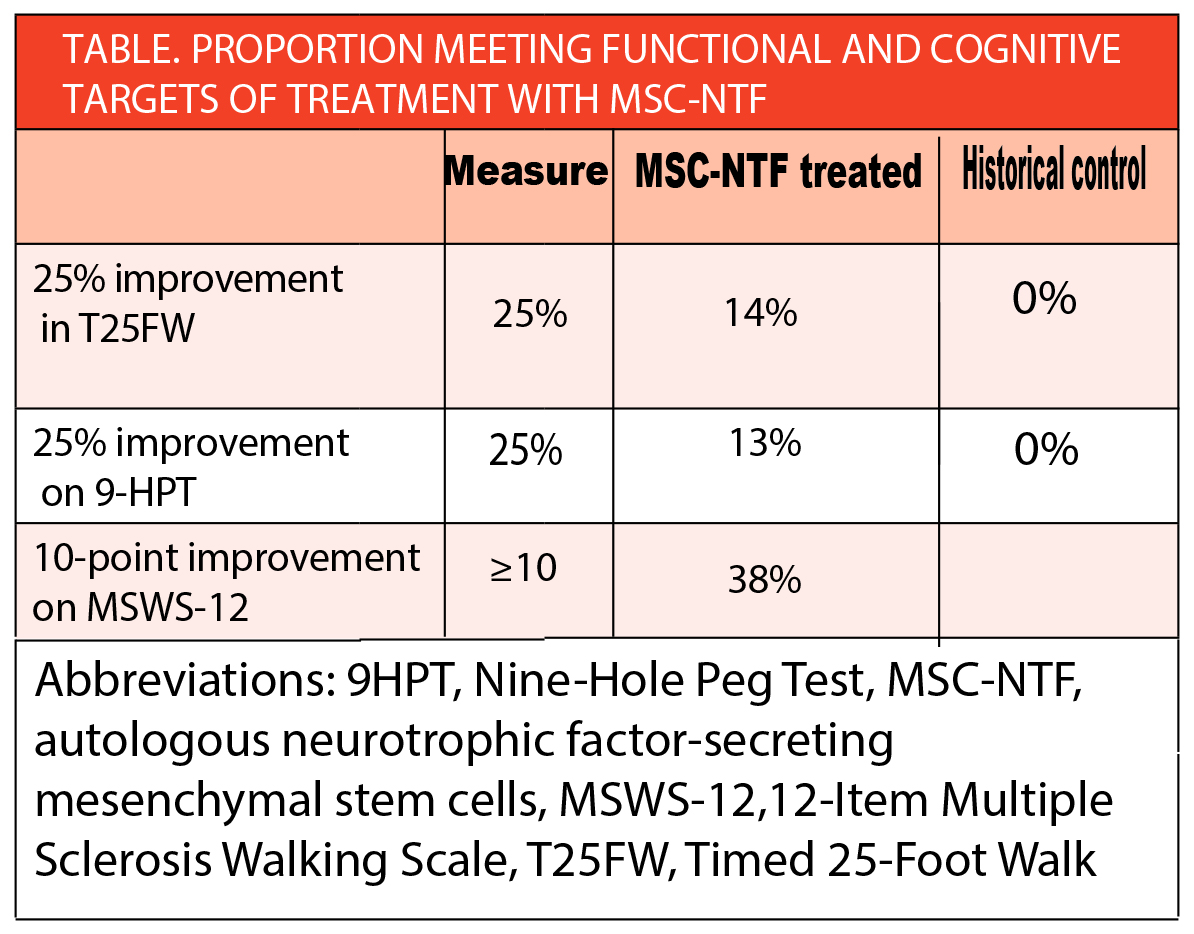
Additionally, 47% of participants had at least an 8-letter improvement across 28 weeks in the low-contrast letter acuity test (LCLA), a visual function test, and 67% showed at least a 3-point improvement in the symbol digit modality test (SDMT), a measure of cognitive processing.
"This is an extremely exciting outcome, as it demonstrates the potential of our proprietary cell therapy NurOwn in progressive MS and expands the body of evidence supporting the NurOwn technology platform in neurodegenerative disease," said Chaim Lebovits, chief executive officer of BrainStorm. "We appreciate the efforts of the Brainstorm team and the commitment and expertise of the clinical trial sites and patients who participated in this important trial despite the challenges imposed by the COVID-19 pandemic. We plan to fully evaluate the results of this study, review the data with the MS scientific community and hopefully continue to advance NurOwn as an important therapeutic option for patients with progressive MS."
The phase 2 clinical trial enrolled 20 participants (56% female) with progressive MS, age18 to 65 (mean=47), with baseline Expanded Disability Status Scale (EDSS) scores 3 to 6.5 (mean=5.4), without evidence of relapse within 6 months of enrollment who were able to walk 25 feet in 60 seconds or less and were permitted to be on a stable dose of disease modifying therapy. Outcomes in the 18 treated participants who completed the trial were compared with a matched clinical cohort (n=48) from the Comprehensive Longitudinal Investigations in MS at the Brigham & Woman's Hospital (CLIMB Study). There were no deaths or adverse events related to MS worsening; 2 participants discontinued the study because of procedure-related adverse events.
