Phase 2 Results for Evobrutinib Suggests Efficacy and Safety for Treatment of Multiple Sclerosis
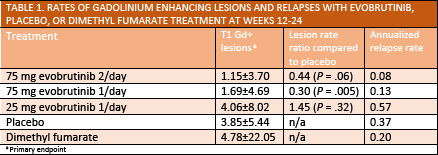
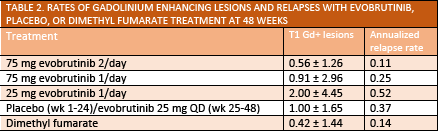
In a double-blind phase 2 study (NCT02975349), adult participants with multiple sclerosis (MS) who were treated with evobrutinib (EMD-Serono, Rockland, MA) (75 mg taken orally once or twice daily) had reduced numbers of T1 gadolinium enhancing (Gd+) lesions as early as 12 weeks after initiating treatment (Table 1). This reduction in T1 Gd+ lesions was maintained through week 48 of the study (Table 2).
Participants treated with evobrutinib also had reduced annualized relapse rate (ARR) compared with those treated with placebo (Table 1), although this did not reach statistical significance, possibly because of study size or the advanced disease state of the participants.
Fernando Dangond, MD, MBA, head of global clinical development in neurology at EMD Serono said, “We are very excited by these data, which encourage us to continue development of evobrutinib. We are proud of the innovative capacity of EMD Serono that led to the in-house discovery and development of evobrutinib, which we hope to bring through clinical trials to help people living with MS.”
Evobrutinib is a novel and highly-specific oral inhibitor of Bruton’s tyrosine kinase, a key regulator of B cell and macrophage functions implicated in MS. In vitro, evobrutinib not only decreases activation of B cells, it also shifts the balance of macrophages from proinflammatory M1 cells to anti-inflammatory M2 cells.
Treatment with evobrutinib appeared well-tolerated throughout the study at all doses with no opportunistic infections, infestations, or lymphopenia. Some participants (5% to 13%) had asymptomatic elevations in alanine aminotransferase (ALT) or aspartate transaminase (AST) after treatment with evobrutinib that were largely driven by events with onset in the first 24 weeks and resolved after stopping treatment.
These data were presented at the American Academy of Neurology annual meeting May 4-10 in Philadelphia, PA, and in more detail published simultaneously in the New England Journal of Medicine (NEJM).
