Phase 2 Results for Atogepant-An Oral CGRP Receptor Antagonist-for Episodic Migraine Prevention
In a phase 2b/3 clinical trial (NCT02848326), adults treated with atogepant (Allergan, Madison, NJ) had greater reduction from baseline in monthly migraine days (MMD) on average, compared with those treated with placebo. No serious adverse events related to treatment occurred. Of those treated with atogepant, the frequency of treatment-emergent adverse events ranged from 57% to 66% across the treatment arms vs 49.5% for those who took placebo. Elevated ALT or AST (>3x the upper limit of normal) occurred in 10 participants (1.8%), balanced across the 6 treatment arms, including placebo.
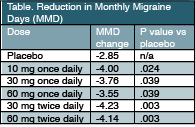
Joel Trugman, MD, clinical development lead for this compound at Allergan noted that, “this first trial, which was a large trial with more than 800 participants, showed promise for atogepant with statistically significant improvement in the number of MMD at all doses. Based on these data, we have advanced 4 of the 5 doses (10, 30, or 60 mg once daily and 30 mg twice daily) into phase 3 trials for people with episodic and chronic migraine. So far, we have been pleased to see good tolerability, and we look forward to sharing the results of those trials at future meetings.”
Results of the phase 2b/3 study were reported at the American Academy of Neurology (AAN) meeting in Philadelphia, PA, May 4-10. Participants were adults with a history of episodic migraine (4-14 MMD) who were randomly assigned to receive atogepant (10, 30, or 60 mg once daily or 30 or 60 mg twice daily; n = 648) or placebo (n = 186) for 12 weeks. Participants were predominantly white (76.1%) women (86.5%) with mean age 40.1. The mean MMD at baseline was 7.67 ± 2.49.
In another study, also presented at the AAN annual meeting, atogepant taken concomitantly with the oral contraceptive ethinyl estradiol/levonorgestrol (EE/LNG) did not significantly alter the pharmacokinetics of EE/LNG, suggesting that treatment with atogepant will not interfere with oral contraception. Participants in this study were postmenopausal or oopherectomized and the treatment with both atogepant and ethinyl estradiol was generally safe and well tolerated.
Atogepant is a novel small molecule antagonist of calcitonin gene-related peptide (CGRP) receptor with high affinity binding to the CGRP receptor and lower affinity to the AMY1 receptor.
