Patient Preferences for Controlling Motor Fluctuations in Parkinson Disease
In a discrete choice study, people with Parkinson disease (PD) weighed what adverse event profile would be acceptable to them if a medication provided a hypothetical number of minutes of ON time.
Respondents generally preferred adjunctive treatment over no treatment at all. When evaluating hypothetical medications with side-effect profiles similar to entacapone, opicapone (Ongentys; Neurocrine Biosciences, San Diego, CA), or no additional medications, 59.4% preferred the opicapone-like profile, 11.3 preferred the entacapone-like profile, and 29.3% preferred no additional medication. Across hypothetical medication profiles participants were willing to tolerate the following:
- 5 more minutes of troublesome dyskinesia for approximately 40 minutes more ON time, however, 10 more minutes of troublesome dyskinesia was not acceptable unless 60 minutes more ON time or more was achieved
- a 10% risk of diarrhea for 40 more minutes of ON time
- A 10% risk of body fluid discoloration for 22 more minutes ON time, although if that risk increased by 30% to 40%, more than 60 minutes more ON time would need to be achieved
- Increasing from 1 pill at bedtime to 1 with every dose of levodopa/carbidopa was considered acceptable in exchange for 22 minutes more ON time
Eiry Roberts, chief medical officer Neurocrine Biosciences, commented, “We know patients have many choices to make and were pleased to complete a study that evaluated patient preferences. It is our hope that these data can be used by neurologists to have open discussions with patients for effective shared decision making about adjunctive treatment.”
These data were generated via an online questionnaire in which people with PD (n=480, mean age 67, 69% diagnosed at least 5 years earlier) were asked 9 questions about their preferences regarding efficacy vs tolerability. The attributes of treatments evaluated are shown in the Table.
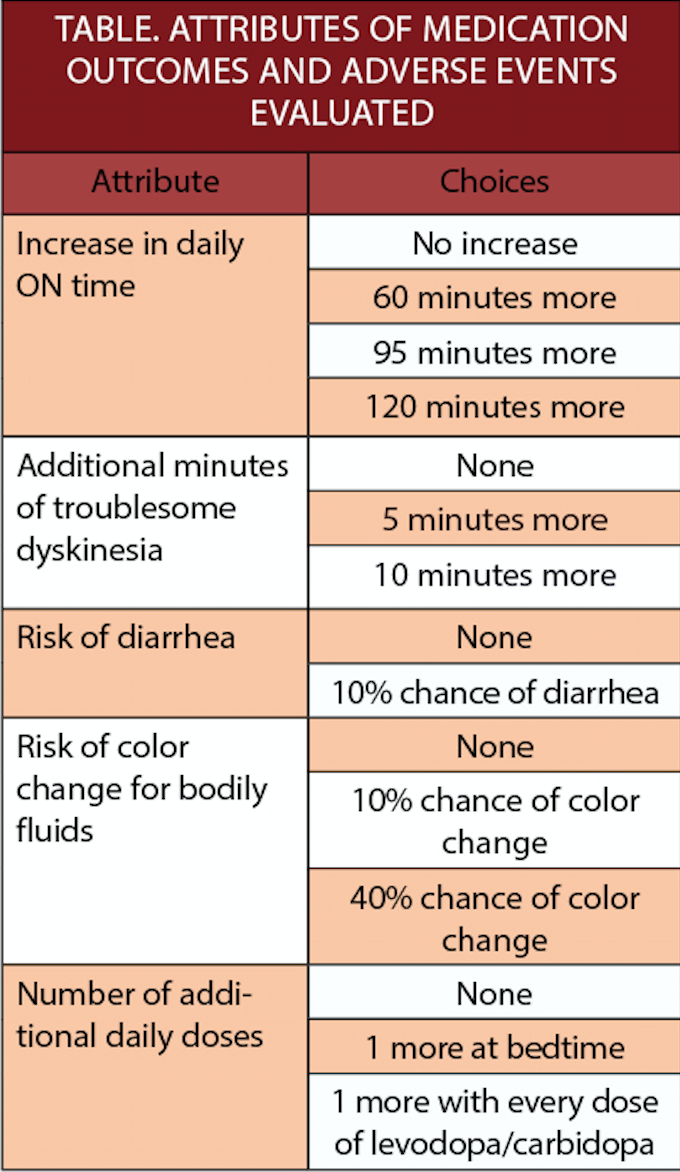
In addition, a 10th question evaluated a choice between medications with a side-effect profile similar to entacapone (dyskinesia, urine discoloration, diarrhea, nausea, hyperkinesis, abdominal pain, and dry mouth) or opicapone (dyskinesia, constipation, increased blood level of creatine kinase increased, low blood pressure/syncope, and decreased weight).
Participants recruited for this study were age 30 to 83, living in the US, and had a self-reported physician-made diagnosis of PD. To be included, participants had to report that they experience OFF episodes and were not taking a medication for cognitive symptoms. The survey was first pretested with semistructured phone or video-conference interviews in a convenience sample of 15 eligible participants.
