Onasemnogene Treatment Results in Independent Mobility and Bulbar Function for Children With Spinal Muscular Atrophy
In the phase 3 SPR1NT study (NCT03505099) children with spinal muscular atrophy (SMA) who received a 1-time treatment of onasemnogene (Zolgensma; Novartis Gene Therapies,East Hanover, NJ) achieved motor milestones. A post-hoc analyses of the START (NCT03421977), STR1VE-EU (NCT03461289), and STR1VE-US (NCT03306277) (n=65) suggested children with SMA Type 1 treated with onasemnogene also achieved bulbar motor function.
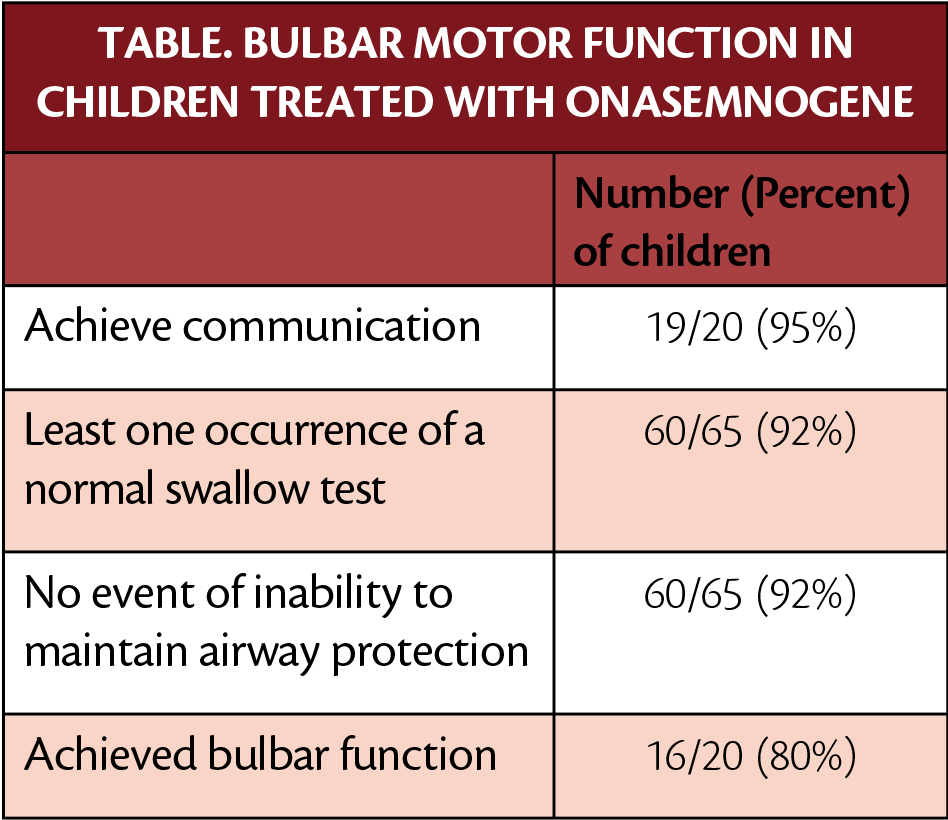
Independent walking was achieved by 93% (14/15) of the children in the 3 post-hoc analyses was able to walk ion their own. From that analysis, 73% of children had of normal development as defined by the World Health Organization (WHO). All 15 children were able to stand alone ≥3 seconds and remained free of feeding tube support and ventilatory support of any kind during the study. In the studies, 80% of the children (16/20) showed bulbar function defined as ability to communicate, swallow, and maintain airway protection.
“Results from SPR1NT again confirm the remarkable impact of Zolgensma for children at risk for SMA who are treated before the onset of symptoms. In sharp contrast to the natural course of SMA, children treated preemptively with Zolgensma are standing and walking, with few or no signs of neuromuscular disease. Many of these children achieve patterns of motor development indistinguishable from their healthy peers without SMA,” said Kevin Strauss, MD, medical director, Clinic for Special Children in Pennsylvania. “These data clearly demonstrate the value of newborn screening for SMA, which is vital to affording children the earliest diagnosis and treatment to ensure the best possible outcomes.”
“The effect of SMA Type 1 on bulbar function often leads to debilitating complications, such as increased risk of aspiration, as well as social consequences from impairment of speech development. These post-hoc data suggest Zolgensma can have an important impact on a child’s well-being,” said Shephard Mpofu, MD, SVP, chief medical officer, Novartis Gene Therapies. “Additional data presented at MDA continue to reinforce the consistent, significant and clinically meaningful therapeutic benefit of Zolgensma in the real-world setting, including in patients outside of our current clinical trial experience.”
No serious treatment-related adverse events were reported in the study.
