Neurotrophic Factor-Secreting Stem Cell Treatment Improves Function in Progressive Multiple Sclerosis
As published in Multiple Sclerosis, off-the-shelf growth factor-secreting stem cell (MSC-NTF) (NurOwn; BrainStorm Cell Therapeutics, New York, NY) treatment of people with progressive multiple sclerosis (MS) improved function compared with case-matched individuals in a registry study.
In the phase 2 trial (NCT03799718) 20 participants, mean age 47 and mean Expanded Disability Status Scale (EDSS) score of 5.4 at screening, were enrolled, and 17 had 3 scheduled treatments with MSC-NTF. Functional measures for these individuals were compared with 48 case-matched participants in the Comprehensive Longitudinal Investigation of Multiple Sclerosis (CLIMB) registry.
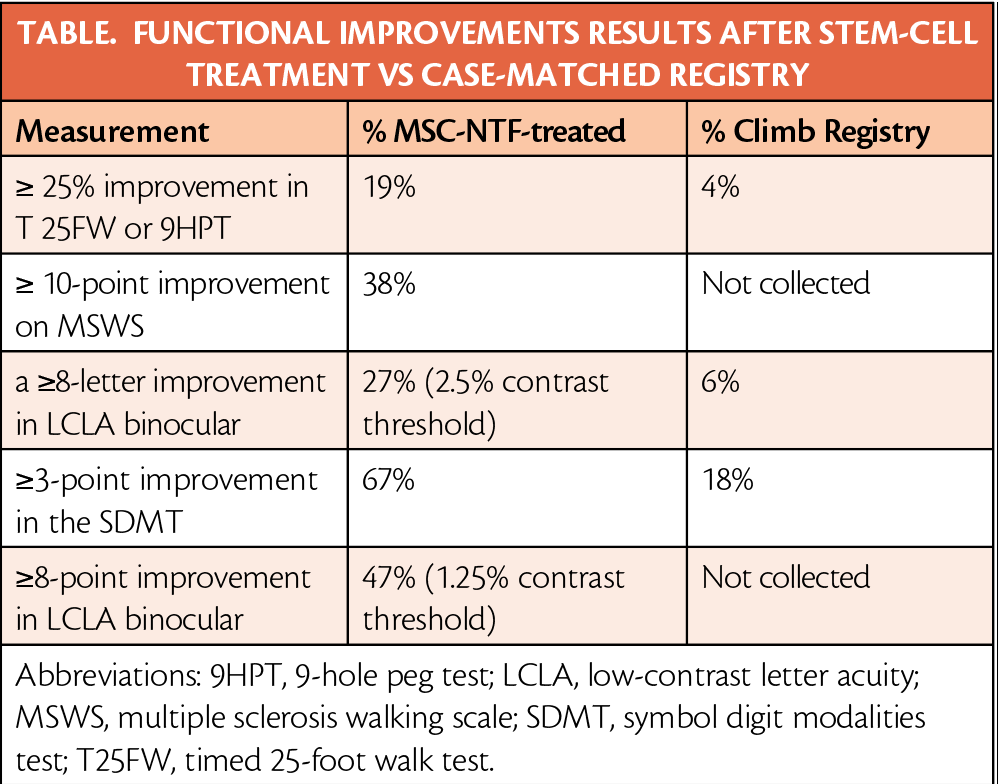
Outcome measures included the timed 25-foot walk speed (T25FW), 9-hole peg test (9HPT), multiple sclerosis walking scale (MSWS), symbol digit modality test (SDMT), and low contrast letter acuity (LCLA) as shown in the Table:
"We were pleased that the study's initial results showed efficacy in patients with progressive MS," said Jeffrey Cohen, MD, Hazel Prior Hostetler endowed chair professor, Cleveland Clinic Lerner College of Medicine, director, Experimental Therapeutics, Mellen Center for MS Treatment and Research, and the paper's lead author. "There are both promising biological and preliminary clinical signals of a treatment effect that will require confirmation in a randomized trial."
In all participants treated with MSC-NTF, functional improvements were seen in LCLA, SDMT and MS Functional Composite (MSFC). Treated participants had mean improvements from baseline of 3.3 points in the LCLA binocular (2.5% contrast), 3.8 points on the SDMT, and 0.18 points in MSFC. In contrast, matched CLIMB registry participants showed declines in function on the LCLA and MSFC.
There were no adverse events related to worsening of MS disease and no clinically significant changes in safety lab results/vital signs. Signs of low back and leg pain developed in 2 participants that were consistent with arachnoiditis after 1 of 3 treatments.
The proposed mechanism of action in progressive MS was supported by increases neuroprotective factors and decreases in inflammatory biomarkers in cerebrospinal fluid.
"There is a high unmet need for better treatments for progressive forms of MS and we congratulate the Brainstorm Cell Therapeutics team for the successful completion and publication of this important study. We look forward to future studies that will help to fully understand the potential of NurOwn and other cell-based therapies for this hard-to-treat form of disease" said Bruce Bebo, executive vice president Research National MS Society.
