Nerinetide May Provide Neuroprotection During Stroke When Tissue Plasminogen Activator (tPA) Not Used
Despite remarkable recent advances in treatment, stroke remains a major cause of mortality and disability. Survival rates have improved such that 33% of people who experienced a stroke can return to work, although with less function than before, and 10% regain most or all function. However, 25% of people who have a stroke die because of it, and 33% receive long-term care. Improving functional outcome after a stroke remains a largely unmet need.
Neuroprotection to prevent or reverse the damage of ischemia-hypoxemia and resulting functional disability is a long-sought and highly elusive goal. Nerinetide is among many agents shown not to have superiority over endarterectomy alone; however, a new post hoc analysis of the clinical trial (NCT02930018) of nerinetide shows that neuroprotection may have occurred in a subset of individuals—those whose treatment did not include tissue plasminogen activator (tPA).
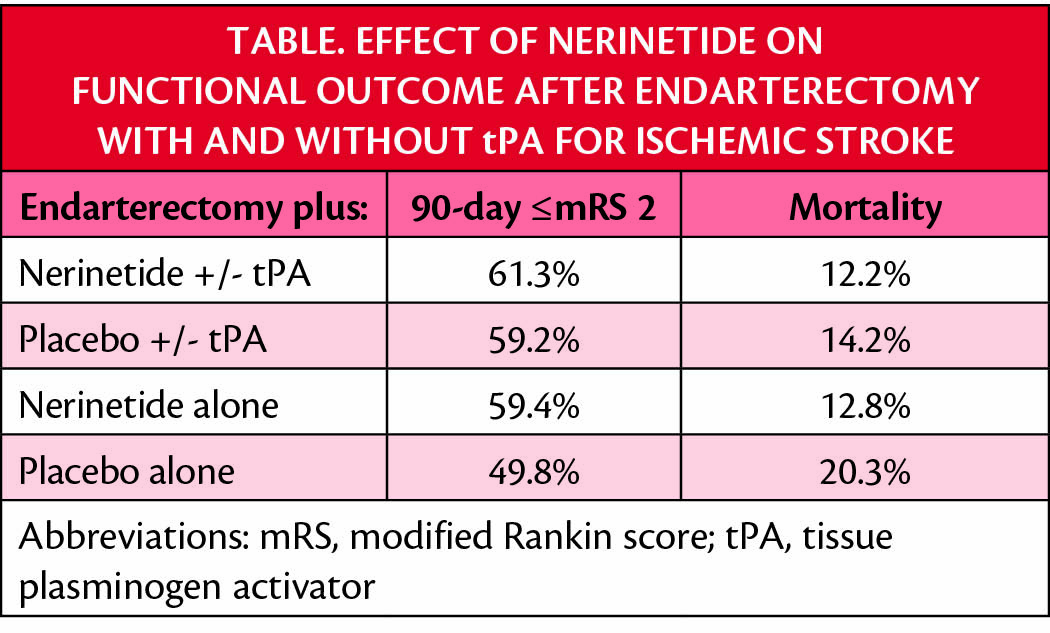
The odds ratio of for achiving a 90-day modified Rankin score (mRS) with nerinetide in the absence of tPA was 1.6 (95% CI, 1.06-2.60; nominal P=.028). Nerinetide treatment in the absence of tPA reduced mortality risk by 43%.
Considering that 4 randomized controlled clinical trials have shown only nominal benefit of tPA in the context of endarterectomy for ischemic stroke, a phase 3 trial (NCT04462536) has begun, which will evaluate nerinetide with endarterectomy in the absence of tPA. The trial has a novel adjustable sample size re-estimation phase planned in which interim results will be assessed by an "arms-length" group that will then advise whether to continue or stop the trial. Other adjustments including digital or deferred consent and telehealth assessment of outcomes are being made to the trial design to improve the ability to complete the trial during the COVID-19 pandemic.
Nerinetide is a small peptide that interacts with, but does not prevent glutamatergic transmission through, N-methyl D aspartate receptors (NMDAR) to inhibit postsynaptic density protein 95 (PSD-95) to prevent activation of neuronal nitric oxide synthetase (nNOS). Inhibition of nNOS lowers nitric oxide (NO) production within neurons. Of note, nerinetide is known to be cleaved and inactivated by plasmin, which is produced by tPA. Blood levels of nerinetide in people with ischemic stroke treated with endarterectomy and alteplase were 60% lower than in those treated with endarterectomy without tPA.
