Natalizumab Treatment Reduced Disability With No Cases of PML in Anti-JC-Virus Antibody Negative Multiple Sclerosis
In the STRIVE clinical trial (NCT01485003),4 years of treatment with natalizumab (Tysabri; Biogen, Cambridge, MA) reduced disability and annualized relapse rates (Table).
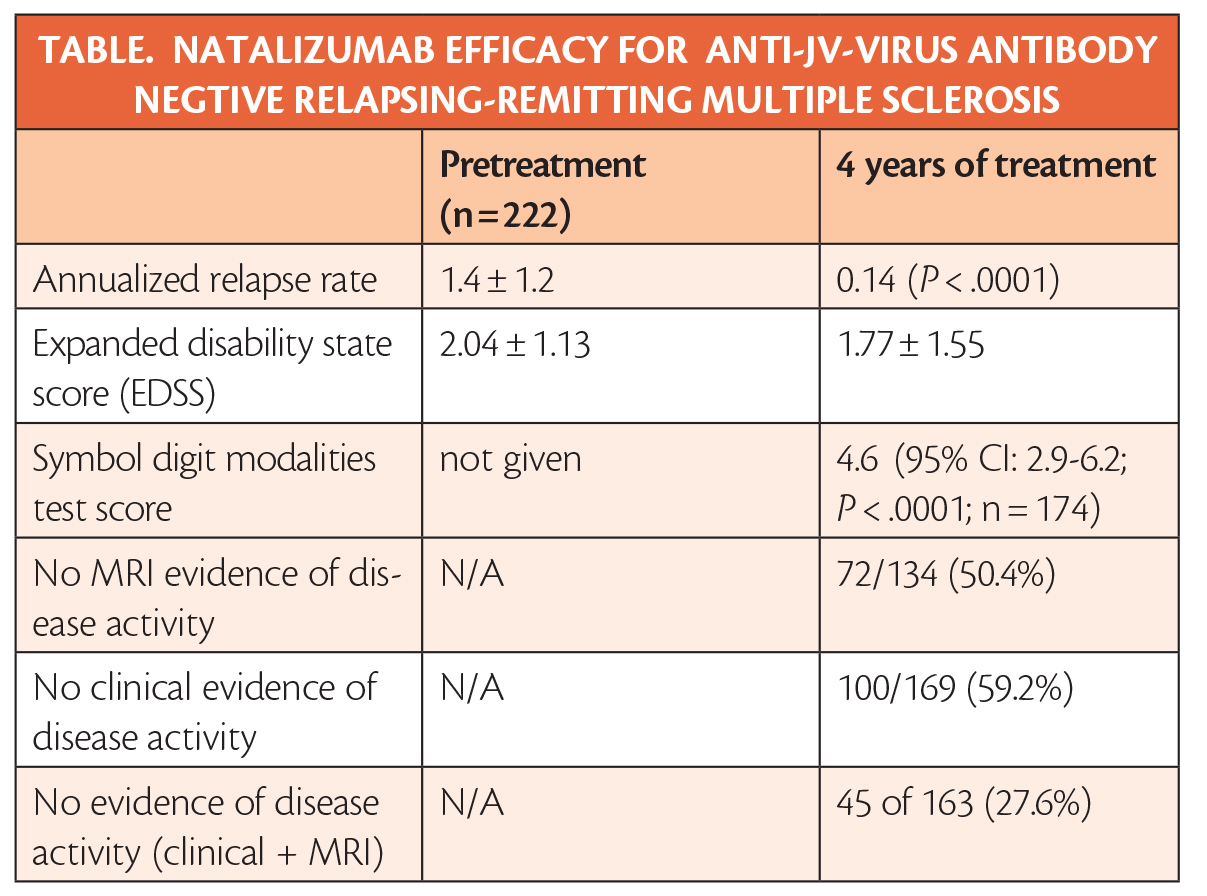
The status of no evidence of disease activity (NEDA) on MRI was reached by 72 of 134 (50.4%) of participants at the end of the study. Clinical NEDA, defined as no relapses and no disability worsening, was achieved by 100 of 169 (59.2%), and overall NEDA (MRI and clinical) was achieved by 45 of 163 (27.6%) of participants. The probability of disability improvement was 43.9% and the probability of disability worsening was 19.3%.
Serious adverse events were reported in 25 of 222 patients (11.3%), with no cases of progressive multifocal leukoencephalopathy (PML).
Participants had received the diagnosis of relapsing-remitting multiple sclerosis (RRMS) less than 3 years before entering the study, and had a mean expanded disability state score (EDSS) of 2.04 +/- 1.13, and an annualized relapse rate (ARR) of 1.4+/-1.2 in the previous year. All participants were verified as negative for anti-JC virus antibodies before starting treatment.
In this study, clinical NEDA was defined as no relapses or EDSS worsening (score increase ≥1.5 from baseline [BL] of 0.0, ≥1.0 from BL of 1.0-5.5, or ≥0.5 from a BL ≥6.0, confirmed over 24 weeks). On MRI, NEDA was defined as no enlarging or new gadolinium-enhanding T2-hyperintense lesions.
These results are being presented at the Congress of the European Committee for Treatment and Research in Multiple Sclerosis in Stockholm, Sweden, September 11-13, 2019.
