Natalizumab Every 6 Weeks Is Effective for Treatment of Multiple Sclerosis and Reduces Risk
Treatment of multiple sclerosis (MS) with 300 mg natalizumab (Tysabri; Biogen, Cambridge, MA) every 6 weeks vs every 4 weeks was evaluated in the phase 3b NOVA study (NCT03689972). Both dosing intervals were found equally effective in participants (n=499) who either switched to 6-week intervals between doses or continued with 4-week intervals for 72 weeks. All participants had stable disease characteristics during a year of treatment with natalizumab at enrollment in this open-label controlled trial.
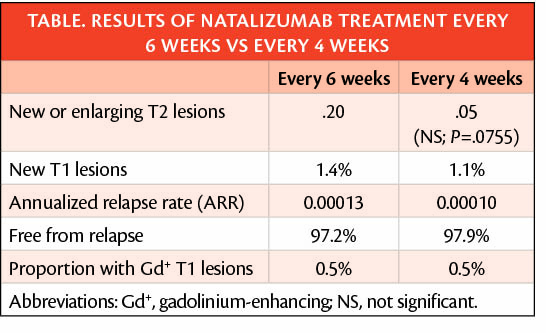
No statistically significant or clinically meaningful difference was seen in the number of new or newly enhancing T2 lesion, although these differed numerically (.20 vs .05; P=.0755 for 6 and 4 week intervals, respectively). This difference is thought to be driven by 2 outliers in the 6-week interval group; 1 had asymptomatic progressive multifocal leukoencephalopathy (PML), and 1 developed lesions 3 months after discontinuing treatment.
PML is a rare and serious brain infection that has been seen to occur with natalizumab treatment. The risk of PML can be mitigated with pretreatment testing for JC virus antibody index and monitoring during treatment. Extending the time interval between doses has also been suggested as a way to decrease risk of PML. Analysis of registry data suggests the hazard ratio for PML with 6-week interval dosing is 0.12 compared with 4-week interval dosing (P<.001). This reported study now provides statistically significant evidence that efficacy of treatment is not diminished by such extended-interval dosing.
“The NOVA study provides the first prospective, randomized efficacy data of every 6-week dosing with natalizumab, building on its well-established clinical profile and the real-world findings. . .” said Maha Radhakrishnan, MD, chief medical officer at Biogen. “In addition to the safety analyses from the TOUCH Prescribing Program, which showed significant reduction in the probability of PML, the results from NOVA deliver a more comprehensive understanding of the 6-week dosing regimen of natalizumab.”
