Lecanemab Reduces Blood Biomarkers of Alzheimer Disease
In a clinical trial (NCT01767311) of lecanemab (BAN2401, Eisai, Woodcliff Lake, NJ) for early Alzheimer disease (AD) reductions in amyloid β (Aβ) were seen. In the double-blind portion of the 65% were amyloid negative at 12 months and 81% of 161 participants, 10 mg/kg biweekly dose, were amyloid negative at 18 months. In an open-label extension, 10 of 12 participants who had not received lecanemab in the double-blind portion, were amyloid negative by 12 months.
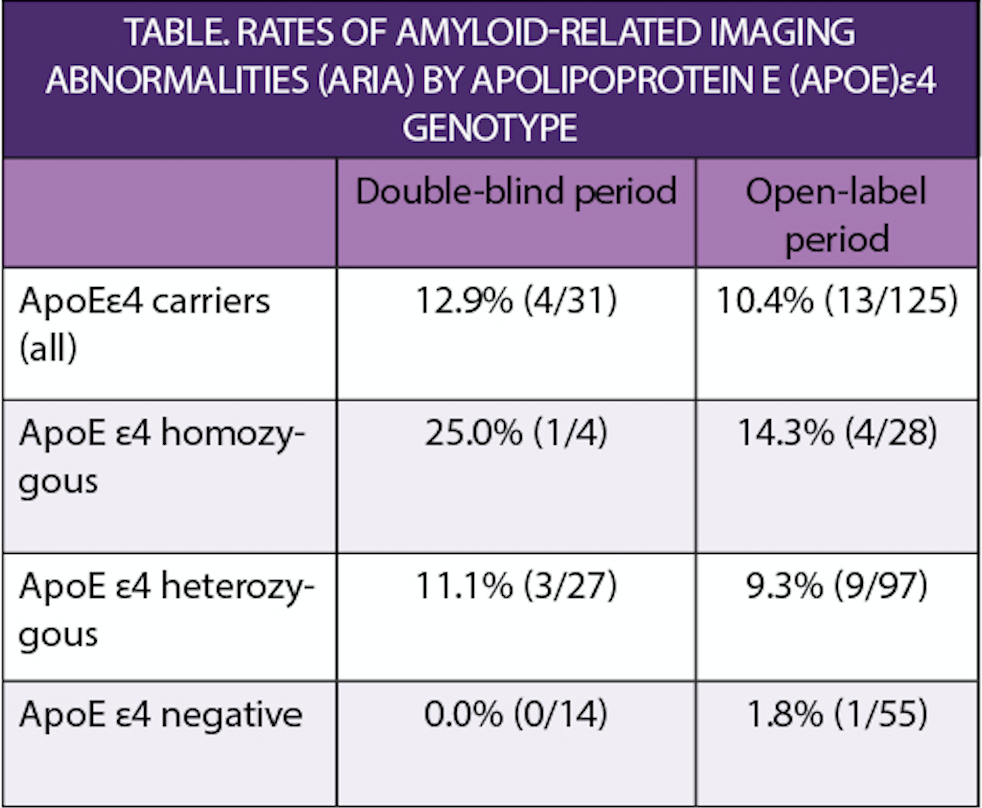
Amyloid was measured with positron emission tomography (PET) imaging. Rates of amyloid-related imaging abnormalities (ARIA) varied with genotype for apolipoprotein E ε4 alleles as shown in the Table. ARIA is an often asymptomatic but sometimes dangerous side effect observed in clinical trials of antibodies to Aβ.
“The invited lecanemab presentations at AD/PD provide new and exciting insights into how the mechanism of action of late-stage antiamyloid antibodies differ and how that may help simplify the patient journey by offering a less frequent dosing regimen while providing long-term benefit,” said Lynn Kramer, MD, chief clinical officer, Neurology Business Group, Eisai. “Eisai aims to bring these potential innovations to people living with early AD and healthcare providers as quickly as possible as we work to fulfill our human health care mission.”
