Istradefylline Reduces OFF Time and Symptoms in Parkinson Disease
Post hoc analysis of pooled data suggests that individuals with Parkinson disease (PD) who had baseline dyskinesia before starting istradefylline (Nourianz; Kyowa Kirin, Bedminster, NJ) were more likely to experience dyskinesia as an adverse event during treatment than those who did not have dyskinesia at baseline. However, both groups on istradefylline experienced a reduction in "OFF" time and an increase in "ON" time without troublesome dyskinesia, regardless of the presence of dyskinesia at baseline. Dyskinesia is recognized as the most frequent adverse reaction in istradefylline treatment for individuals experiencing "OFF" episodes. These findings were presented during the 6th Congress of the European Academy of Neurology (EAN).
The pooled analysis examined subgroup data from 2,719 participants with PD taking levodopa-containing products with or without other antiPD medications, who experienced "OFF" episodes, either with baseline dyskinesia (+BL-dyskinesia) or without baseline dyskinesia (–BL-dyskinesia) prior to the addition of istradefylline or placebo to their treatment regimen. Data were pooled from 8 randomized, placebo-controlled, double-blind clinical studies. In the studies, participants received a daily 20 mg or 40 mg dose of istradefylline or a placebo for 12 or 16 weeks. Primary analyses from these studies showed that the use of istradefylline as an adjunctive treatment to levodopa/carbidopa in participants with PD experiencing "OFF" episodes was associated with a decrease in "OFF" time and an increase in "ON" time without troublesome dyskinesia.
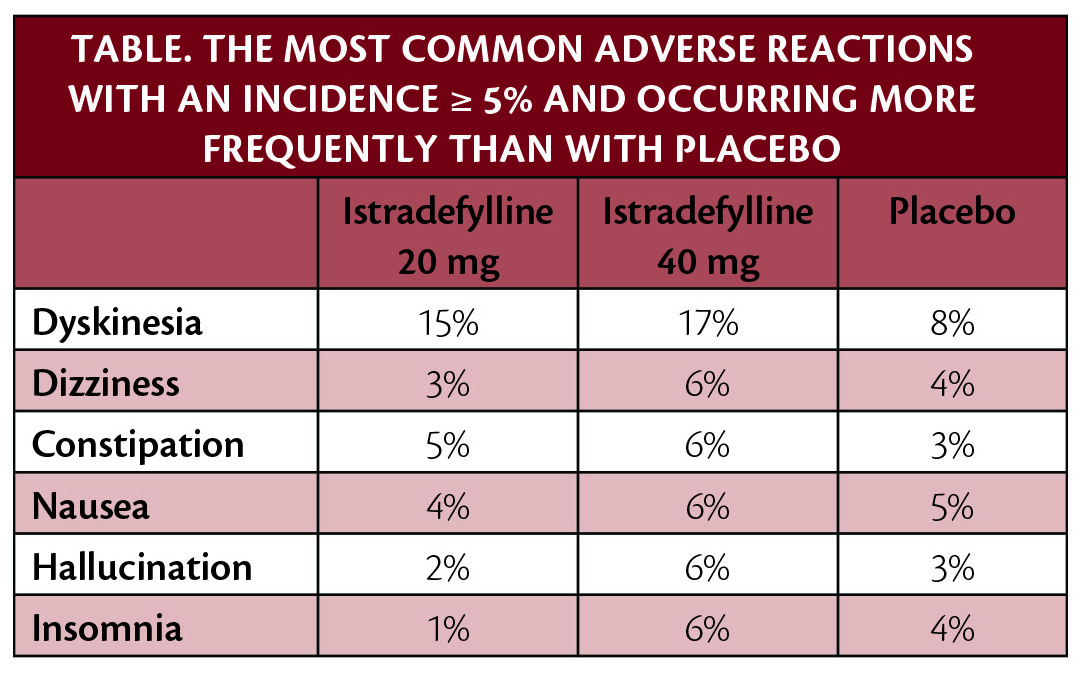
Results from the post hoc subanalysis of the 8 pooled studies demonstrated that dyskinesia was reported by more participants as an adverse event during the trials by participants with baseline dyskinesia vs participants without baseline dyskinesia, regardless of whether they received istradefylline or placebo. Dyskinesia as an adverse event during the studies was reported by 14.1%, 24.7%, and 25.9% of participants with baseline dyskinesia (+BL-dyskinesia) receiving a placebo or 20 mg/day or 40 mg/day of istradefylline, vs 3.5%, 4.4% and 8.4% of participants without baseline dyskinesia. Reduction in "OFF" time and increase in "ON" time without troublesome dyskinesia were greater with istradefylline than with placebo, which was consistent with previous studies.
Although the data adds to the understanding of dyskinesia as an adverse event in the clinical trials, physicians should continue to monitor individuals for dyskinesia or exacerbation of existing dyskinesia during istradefylline treatment.