Investigational MS Treatment Diroximel Fumarate Has Improved Gastrointestinal Tolerability
Topline results from the EVOLVE-MS-2 study (NCT02634307), of diroximel fumarate (ALKS8700, BIIB098; Alkermes, Waltham, MA and Biogen, Cambridge, MA) for treatment of relapsing-remitting multiple sclerosis (RRMS) have been announced. Participants treated with diroximel fumarate had significantly fewer days of gastrointestinal (GI) symptoms with intensity scores of 2 or more on the Individual Gastrointestinal Symptom and Impact Scale (IGISIS) compared with participants treated with dimethyl fumarate (Tecfidera; Biogen, Cambridge, MA) (P = .0003). Participants treated with either drug self-reported key GI symptom intensity twice daily.
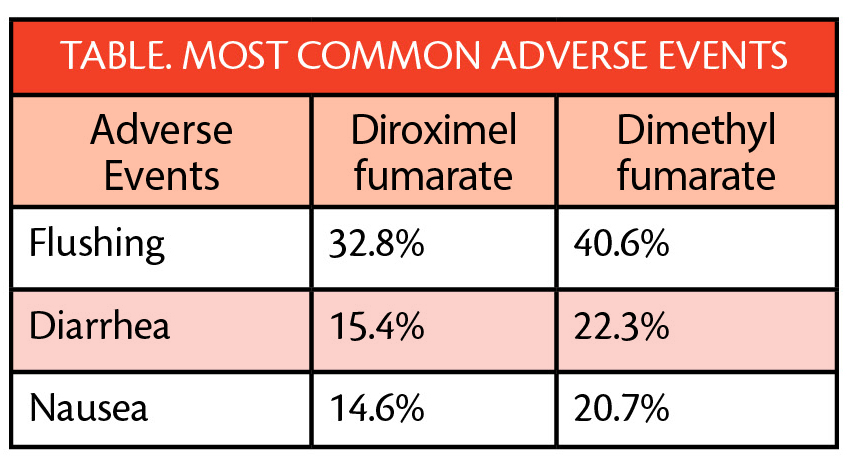
Of those treated with diroximel fumarate, 1.6% left the study because of overall adverse events (AEs) and 0.8% left because of GI AEs specifically. In contrast, 6.0% of those treated with dimethyl fumarate left the study because of overall AEs and 4.8% specifically for GI AEs. Adverse events (AEs) reported for both treatment groups included flushing, diarrhea, and nausea (Table).
“With a chronic disease like MS, interrupting or stopping treatment due to GI side effects can often provoke the return of disease activity. Physicians and patients should work together to choose a medication that provides the right balance of efficacy, safety and tolerability to help manage patients’ MS and meet their treatment goals,” said Robert Naismith, MD, professor of neurology, Washington University School of Medicine in St. Louis. “These topline results suggest that diroximel fumarate offers a differentiated GI tolerability profile and may represent an important new option for people living with relapsing MS.”
In the EVOLVE-MS-2 trial, a 5-week study, 506 participants with RRMS were randomly assigned to receive treatment with 462 mg daily of diroximel fumarate or 240 mg twice daily of dimethyl fumarate. Severity and duration of GI symptoms were reported using the IGISIS rating scale that ranges from 0 (not at all) through 10 (extreme) and includes nausea, vomiting, upper and lower abdominal pain, and diarrhea.
Diroximel fumarate is an investigational, novel oral fumarate with a distinct chemical structure under review by the Food and Drug Administration (FDA) with a PDUFA (Prescription Drug User Fee Act) target action date in the fourth quarter of 2019. Biogen intends to market diroximel fumarate under the conditionally approved brand name Vumerity.
