Inebilizumab Reduced Severity of Neuromyelitis Optica Spectrum Disorder Attacks
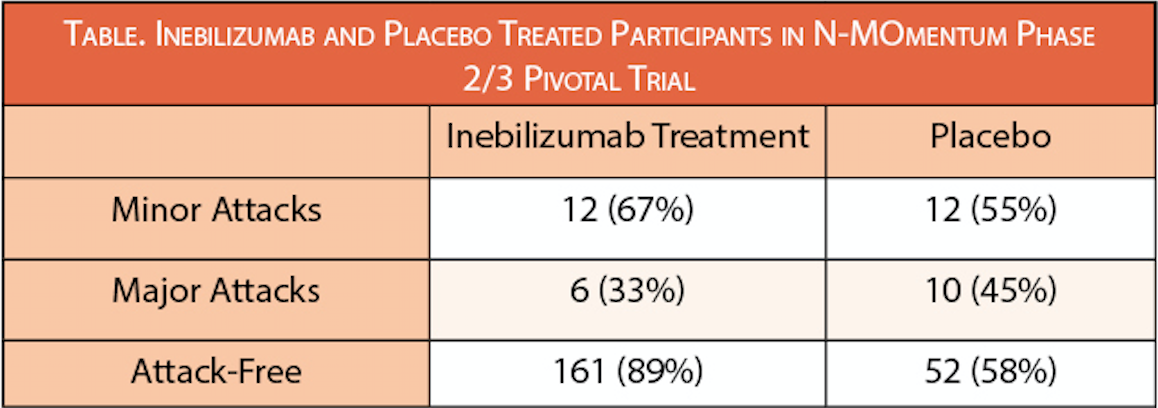
A new analysis shows treatment with inebilizumab (Uplizna; Horizon Therapeutics, Deerfield, IL) effectively reduced the severity of neuromyelitis optica spectrum disorder (NMOSD) attacks. In the study (NCT02200770), NMOSD attacks were graded as major or minor based on changes in neurologic function according to a modified Opticospinal Impairment Scale (OSIS).
“Acute attacks in NMOSD can lead to irreversible consequences and permanent disability in patients, making targeted treatments important to limit disease activity and its severity in patients,” said Jeffrey Bennett, MD, PhD, University of Colorado and study author. “This analysis illustrates the clear clinical impact of Uplizna to reduce the debilitating effects of disease while contributing to our understanding of the link between serum biomarkers and severity of attacks.”
The analysis also evaluated the relationship between the severity of these attacks and biomarkers of disease activity, such as serum glial fibrillary acidic protein (sGFAP) and serum neurofilament light (sNfL). sGFAP levels were significantly higher during major vs minor attacks overall (P=.023) and trended higher for optic neuritis (ON-specific attacks (n=20, P=.06).
sNfL levels were higher for major vs minor attacks overall (P=.032), although the levels did not correlate with the severity of ON attacks. In participants who had attacks during the randomized controlled portion of the trial, sNfL levels were higher among those treated with placebo vs inebilizumab at week 26 (P=.03).
“. . .the Uplizna clinical trial demonstrated that a majority of patients were attack-free after being treated, it also offered an increased understanding of how Uplizna impacted the severity of the attacks that occurred,” said Kristina Patterson, MD, PhD, medical director, neuroimmunology, Horizon. “This is an important consideration for physicians as they evaluate treatment options for their patients, as severe attacks can have debilitating consequences.”
Inebilizumab is the first and only B-cell-depleting agent approved by the Food and Drug Administration (FDA) for the treatment of NMOSD in adults who are antiaquaporin-4 (AQP4) antibody positive.
