Gene Therapy for Huntington Disease Was Safe and Decreased Biomarkers in Clinical Trial
In a phase 1 /2 clinical trial (NCT04120493), 10 participants with early stage Huntington disease were treated with a low dose of gene therapy (AMT-130; uniQure, Lexington, MA) delivered to the striatum in a MR-guided neurosurgical procedure or a sham procedure. All participants had between 40 and 44 CAG repeats in the huntingtin gene, with Total Functional Capacity scores of 10 to 13, and Total Motor Scores of 7 to 23 at baseline. No serious adverse events related to treatment occurred.
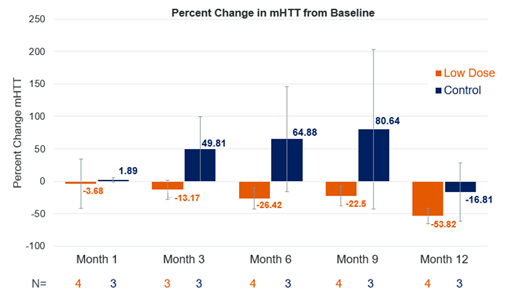
All participants had cerebrospinal fluid (CSF) analysis to measure neurofilament light chain (NfL) and mutant huntingtin protein (mHTT). There was no measurable mHTT at baseline in 2 of 6 participants treated with gene therapy and 1 of 4 treated with the sham procedure.
Among those with measurable mHTT levels at baseline, those treated with gene therapy vs the sham procedure had a mean reductions in mHTT levels of -53.8% (range, -77% to -41%) vs -16.8% (range, -47% to +35%) at 12 months. In the 3 individuals with measurable mHTT at baseline who had sham treatment, there were increases from baseline in mHTT levels at 1, 3, 6, and 9 months.
“We are encouraged by this 12-month update on the patients enrolled in the low-dose cohort,” stated Ricardo Dolmetsch, PhD, president of research and development at uniQure. “Thus far in the clinical trial, AMT-130 has been well-tolerated, with no serious adverse events related to the gene therapy and NfL levels approaching baseline. We are also pleased to observe trends suggesting target engagement that are supported by the lowering of mHTT protein in evaluable patients receiving AMT-130. We look forward to presenting additional clinical data, including functional outcomes, on all patients from this important study next year.”
NfL levels increased initially in those treated with gene therapy, peaking at 1 month and then decreasing to a mean of 8% above baseline (range -14% to +46%). In the 4 participants given sham treatment, the mean NfL level was stable or slightly increased (range -35% to -1%).
Serious adverse events occurred that were judged unrelated to treatment. A participant had a deep-vein thrombosis in the elbow that resolved with anticoagulant treatment, and another had transient postoperative delirium.
A cohort of 16 participants have enrolled to be the high-dose cohort in this study and a phase 1/2 study is also ongoing in Europe. Together the aim of these trials is to establish safety, proof of concept, and an optimal dose for potential phase 3 trials.
