Ganaxolone Reduces Seizure Frequency in Tuberous Sclerosis Complex
In an open-label phase 2 trial (NCT04285346), ganaxolone (Marinus Pharmaceuticals, Radnor, PA) treatment of 23 participants with seizures associated with tuberous sclerosis complex (TSC) reduced seizure frequency. Ganaxolone treatment resulted in a median 16.6% reduction in 28-day seizure frequency relative to the 4-week baseline period.
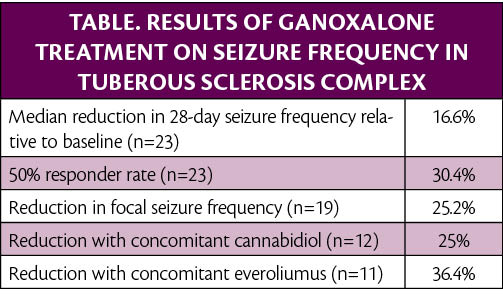
“We believe the totality of the data is encouraging and supports advancing to phase 3. There was notable activity in focal seizures, a meaningful 50% response rate, and consistent results in this refractory patient population, including patients on both cannabidiol and everolimus,” said Joseph Hulihan, MD, chief medical officer of Marinus. “We look forward to initiating our phase 3 trial and adding to the body of evidence that supports ganaxolone’s potential as an innovative treatment option for rare epilepsies.”
The CALM trial enrolled 23 participants from the ages of 2 to 32 years that underwent a 4-week baseline period followed by a 12-week treatment period. Participants received up to 600 mg of ganaxolone 3 times a day. Participants who met eligibility criteria continued treatment during a 24-week extension.
