Fremanezumab Effective for Previously Untreatable Migraines
In the international double-blind placebo-controlled FOCUS study (NCT03308968), participants with episodic or chronic headache documented as inadequately treated with 2 to 4 different classes of medication had clinically meaningful relief from treatment with fremanezumab (Ajovy; Teva, Parsipanny, NJ) vs placebo (Table).
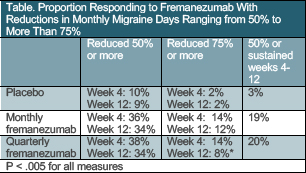
Fremanezumab is a monoclonal antibody to calcitonin gene-related peptide (CGRP) proven effective for prevention of migraine in adults. This study is the first to specifically show that a monoclonal antibody to CGRP is effective for treating headaches that were unresponsive to previously available treatments.
The monthly dosing regimen used was 1 subcutaneous injection of 675 mg in month 1 followed by monthly subcutaneous injections of 225 mg and the quarterly treatment was 1 injection of 675 mg followed by monthly placebo injection.
“Migraine continues to be a difficult-to-treat disease and as the migraine treatment landscape evolves, we remain committed to improving the lives of patients,” said Hafrun Fridriksdottir, executive vice president, global R&D, Teva.
