Fenfluramine Reduces Seizures Regardless of Concomitant Antiseizure Medications
Post hoc analysis of data from (NCT02682927, NCT02826863, NCT02926898) investigated potential interactions of fenfluramine (Zogenix, Emeryville, CA) with other antiseizure medications (ASMs). Efficacy of fenfluramine with concomitant ASMs was also seen.
Fenfluramine treatment provided significant seizure reductions for children, age 2 to 19 with Dravet syndrome, when taken with concomitant antiseizure medications (ASMs) (Table).
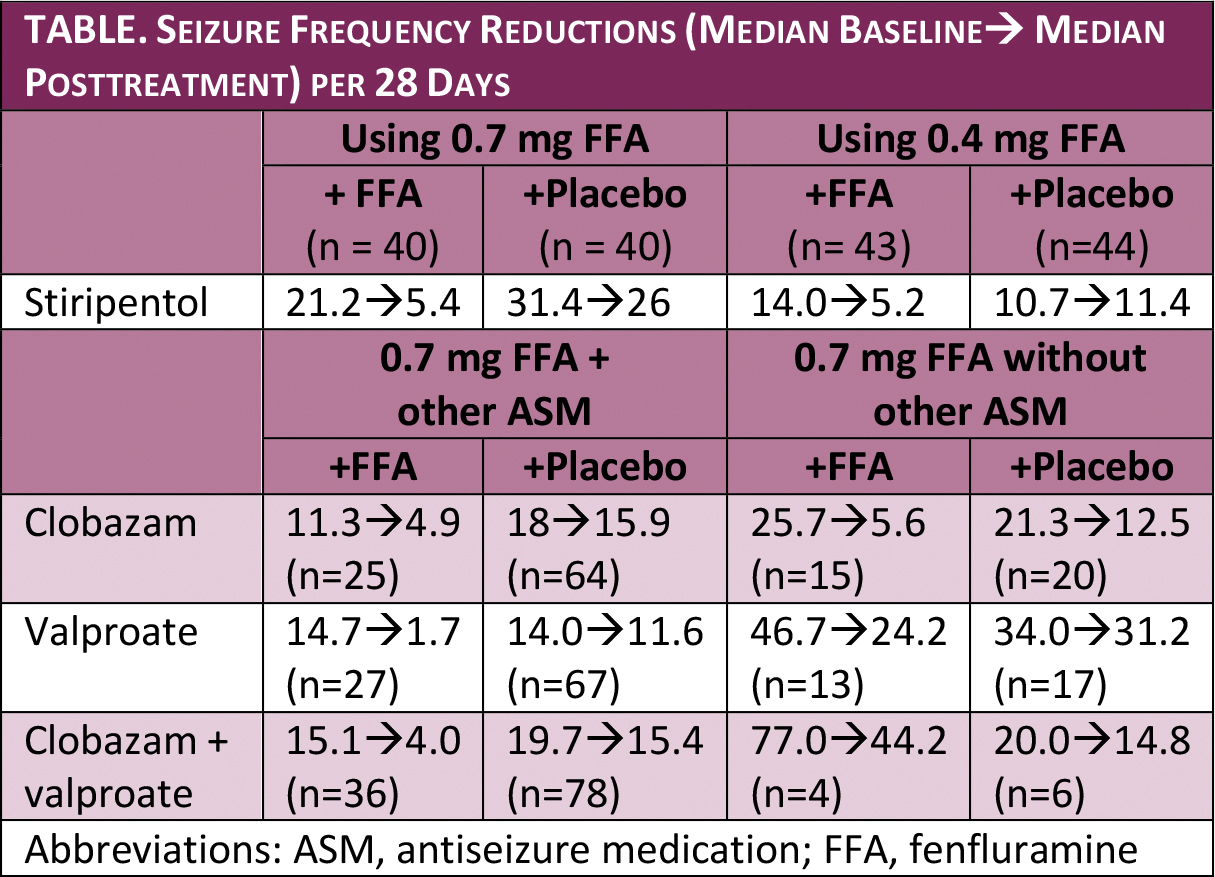
The reductions seen for fenfluramine with and without clobazam or stiripentol were similar, although starting from different baseline seizure frequencies. Children taking both valproate and fenfluramine, however, seemed to have greater reductions than with either drug alone, although the cohorts were small, and this should be interpreted with caution.
Kelly G. Knupp, MD associate professor, University of Colorado Denver - Anschutz Medical
Campus in Aurora, CO and lead author of this analysis said, “With fenfluramine, we have seen greater reductions in seizure frequency. This is a syndrome that is both difficult and essential to diagnose and treat appropriately because certain ASMs can worsen seizure frequency in Dravet syndrome. If approved, fenfluramine will give us another tool to provide early and appropriate treatment for children with Dravet syndrome.”
These results were presented at the American Epilepsy Society Annual Meeting in Baltimore, MD December 6-10, 2019. As previously reported, results for fenfluramine reported at the meeting include that children whose seizures were reduced by 75% also had clinically meaningful improvement in executive function.
Orrin Devinsky, MD, director of the New York University Comprehensive Epilepsy Center and the Saint Barnabas Institute of Neurology and Neurosurgery and professor of Neurology, Neurosurgery, and Psychiatry at NYU Langone School of Medicine said, “Fenfluramine provides a robust and clinically meaningful decrease in seizure frequency relative to placebo and also increased the number of days between seizures from a median of 9.5 days with placebo to 25 days with fenfluramine treatment (P=.001). This shows that fenfluramine is not just an effective drug for Dravet syndrome, it is a very effective drug. More seizure-free days can potentially improve quality of life by increasing the number of days that children are functioning and able to benefit from therapy and education. I’m hopeful that this treatment, if approved, represents a new era for care of children with Dravet syndrome.”
No evidence of cardiovascular adverse events was seen, and no adverse drug-drug interactions were observed. The most common treatment-emergent adverse events (TEAEs) with fenfluramine vs placebo seen in the blinded period of these studies were fatigue (38.5% vs 15.5%), somnolence (28.7% vs 8.3%), lethargy (33.6% vs 10.7%) and appetite suppression and hypophagia (36.9% vs 8.3%).
