Fenfluramine Consistently Reduces Seizures in Children and Young Adults with Dravet Syndrome
A third phase 3 study (NCT02926898) further validates the significant reduction of convulsive seizures seen when individuals with Dravet syndrome (DS) receive fenfluramine (Fintepla; Zogenix Inc., Emeryville, CA). Fenfluramine is an oral treatment for seizures associated with Dravet syndrome (DS) in individuals age 2 years and more. Participants with the lowest dosage of fenfluramine at 0.2 mg/kg/day had a 49.9% monthly mean seizure reduction increase. Whereas participants with a dose of 0.7 mg/kg/day had an increase of 64.8% compared with placebo.
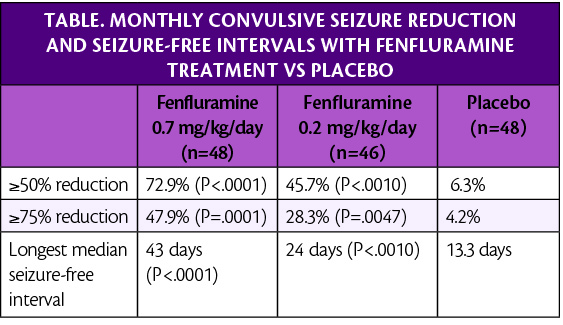
Participants received 0.7 mg/kg/day (26 mg maximum daily dose; n=49), 0.2 mg/kg/day of fenfluramine (26 mg maximum daily dose; n=49, n=46, respectively), or placebo (n=48). The dose was titrated up over a 2-week period, which was followed by a 12-week maintenance period. Participants treated with 0.7 mg/kg/day of fenfluramine experienced a median seizure frequency reduction of 73.7% compared with 7.6% in those treated with placebo (P<.0001).
This third study (Study 3) was a randomized double-blind placebo-controlled phase 3 trial in 143 participants with DS, age 2 to 18 years. Participants mean monthly seizure frequency was 69/ month, and participants were not taking any medication that was effectively managing their seizures before the study. After a 6-week observation period, the participants were placed in 3 groups based on different dosages.
The most common adverse reactions, similar to the first 2 phase 3 studies of fenfluramine (NCT02682927, NCT02826863), were decreased appetite, somnolence, sedation, lethargy, diarrhea, constipation, abnormal echocardiogram, fatigue, malaise, asthenia, ataxia, and balance disorder.
Over 91% of participants treated with fenfluramine had at least 1 treatment-emergent adverse event compared with 83.3% of those treated with placebo. Serious adverse events were similar in all groups; 3 participants treated with fenfluramine and 2 treated with placebo had at least 1 treatment-emergent serious adverse event, and a participant taking placebo died of sudden unexpected death in epilepsy (SUDEP).
