FDA Clears Neurostimulator System for Acute Treatment of Migraine
The Food and Drug Administration (FDA) gave clearance for a noninvasive multichannel brain neuromodulation system (Relivion; Neurolief, Tampa, FL) for acute migraine treatment. The neuromodulation system is worn as a headset and offers precise, personalized care to individuals. The treatment is delivered by stimulation to 6 branches of the occipital and trigeminal nerves via 3 adaptive output channels.
The FDA marketing clearance is based on the results of a multicenter prospective randomized double-blind placebo-controlled clinical RIME study (NCT03631550). Participants in the study were placed in 2 groups; the active group was treated with the neuromodulation system and the control group with a placebo device. The 2 groups were compared by the achievement of pain freedom within 2 hours and freedom of most bothersome symptoms (MBS), such as phonophobia, photophobia, or nausea.
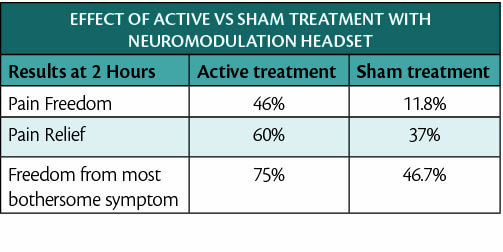
“The FDA clearance of the Relivion noninvasive device is an important event for those suffering from migraines, as it is the only neuromodulation technology thus far that has demonstrated statistically significant efficacy in providing complete freedom of migraine symptoms within 2 hours after treatment, in a sham-controlled clinical trial,” said Stewart J. Tepper, MD, professor of Neurology at the Geisel School of Medicine at Dartmouth, who was the principal investigator on the pivotal international trial. “Patients will now have access to a highly effective, easy-to-use, noninvasive, and drug free therapeutic option that will help them regain control of their lives.”
No serious adverse events were reported. Participants started using either the neuromodulation system or a placebo device with lower treatment intensity, at the onset of their migraine episode for up to 1 hour.
