FDA Approves Apomorphine Sublingual Film for Treatment of OFF Periods in Parkinson Disease
The Food and Drug Administration has approved apomorphine sublingual film (Kynmobi; Sunovion, Marlborough, MA) for the acute, intermittent treatment of “off” episodes in patients with Parkinson disease (PD). This new formulation of apomorphine provides convenient administration (placing a film under the tongue) without requiring assembly or administration of an injection or inhalation device. For the estimated 60% of people with PD who have OFF periods—whether predictable or unpredictable—and caregivers, this may provide a simpler way to treat OFF episodes whenever and wherever they occur.
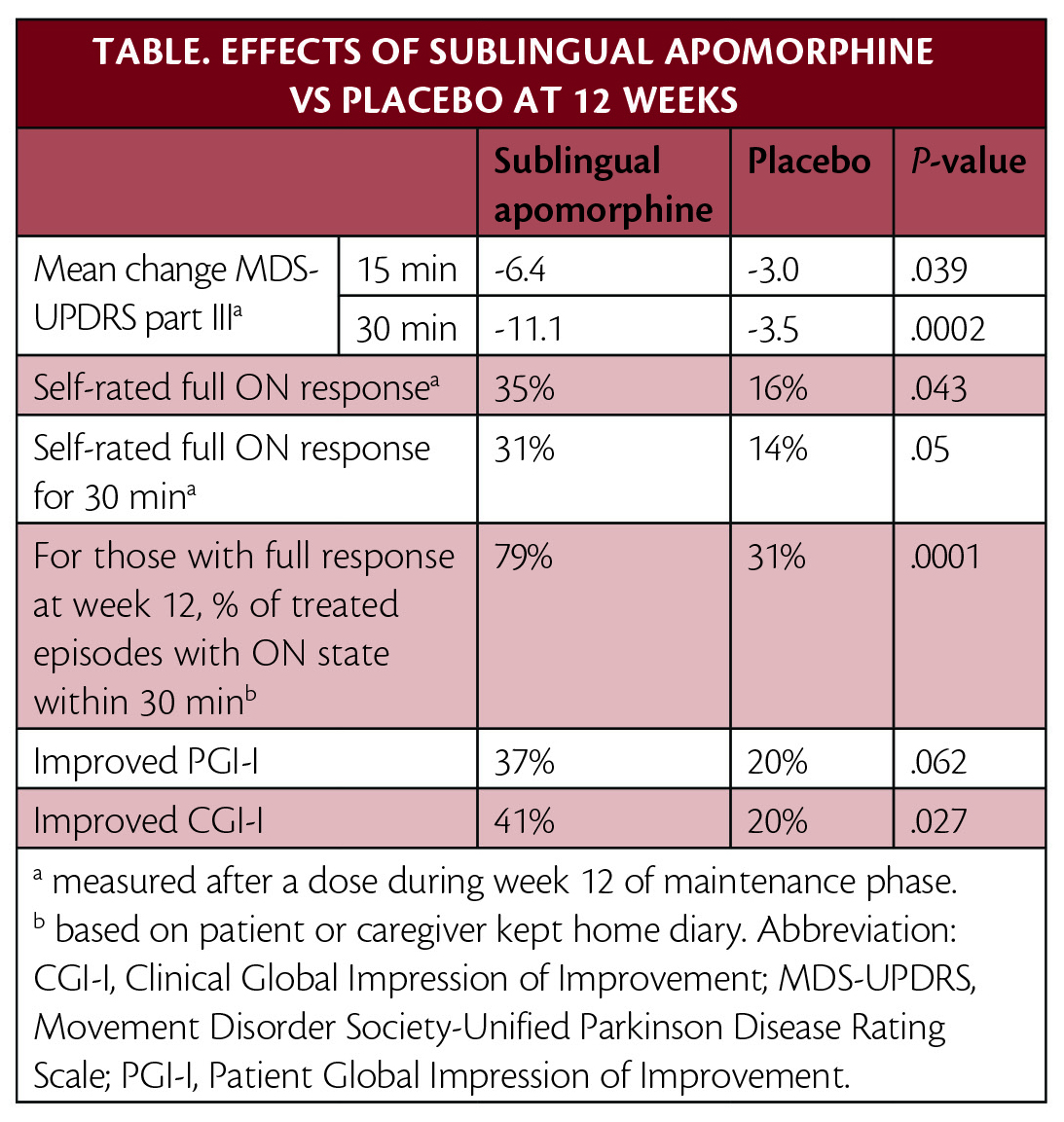
FDA approval was based on multiple clinical trials. In the pivotal phase 3 clinical trial (NCT02469090), published in Lancet Neurology, treatment with apomorphine sublingual film provided statistically significant and clinically meaningful improvement in motor symptoms vs placebo (Table).
Rob Goldman, PhD, head of Global Clinical Research and Medical Affairs of Sunovion noted, "We are pleased to bring to clinicians another tool to treat OFF episodes, helping patients rapidly return to an ON state wherever and whenever an OFF episode occurs. The ability to control motor symptoms quickly may also help people with PD to perform their activities of daily living."
In clinical studies, adverse reactions led to discontinuation in 9% of patients in the titration phase, and 28% of patients in the maintenance phase, compared with 7% of patients on placebo (in the maintenance phase). The most common adverse reactions leading to discontinuation during the maintenance phase were oral/pharyngeal soft tissue swelling, oral mucosal erythema, and nausea/vomiting. A single participant with known cardiac risk factors died of a cardiac arrest during the trial.
Upon enrollment, participants were levodopa-responsive, experienced at least 2 hours of OFF time per day, and had a mean of 4 OFF episodes per day. The effective dose for each participant was determined in a titration period. Participants began treatment with 10 mg, which was increased in 5 mg increments until a dose that resulted in an ON state and was tolerable (maximum 35 mg) was reached. Participants were then randomly assigned to take that dose (n=54) or placebo (n=55) as needed up to 5 times daily for 12 weeks.
