FDA Approves Ravulizumab for Generalized Myasthenia Gravis
The Food and Drug Administration (FDA) approved ravulizumab (Ultomiris; Alexion, Wilmington, DE) for the treatment generalized myasthenia gravis (gMG) in adults who are antiacetylcholine receptor antibody-positive (AChR+). In the CHAMPION-MG trial (NCT03920293), participants treated with ravulizumab showed improvement in activities of daily living. Ravulizumab has potential to reduce treatment burden with dosing every 8 weeks
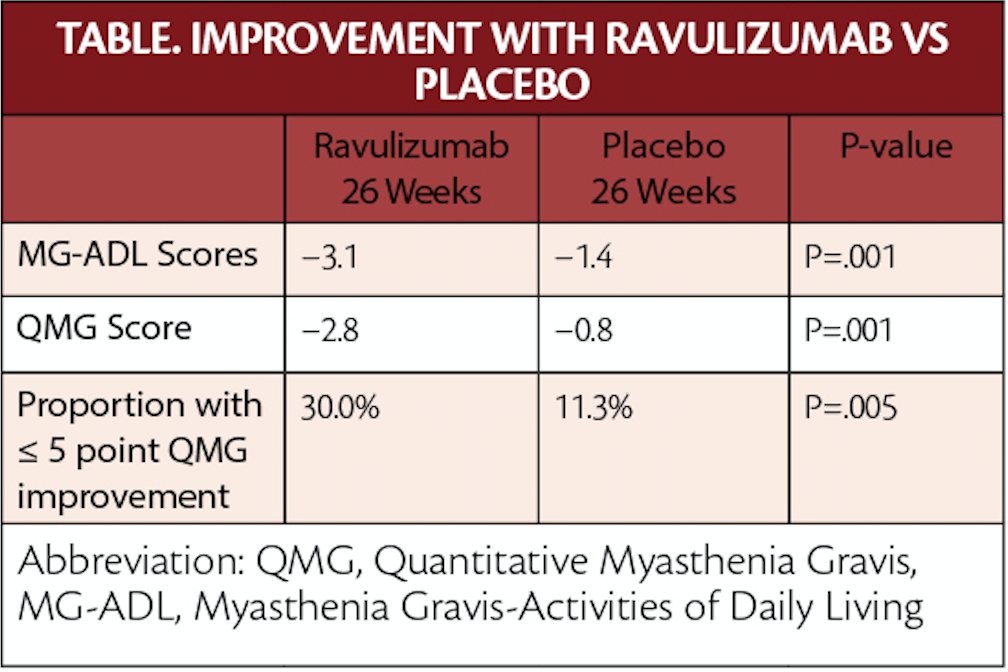
In the CHAMPION-MG phase 3 trial, ravulizumab was superior to placebo when comparing the change from baseline in the Myasthenia Gravis-Activities of Daily Living Profile (MG-ADL) total score at 26 weeks.
Professor James F. Howard, Jr, MD, Department of Neurology at The University of North Carolina School of Medicine and lead primary investigator in the CHAMPION-MG trial said, “Despite recent advances, managing gMG is complex. Earlier intervention can preserve function and quality of life. This approval offers patients, including those with milder symptoms, a long-acting C5 inhibitor with early onset and reliable efficacy.”
The most common adverse events were upper respiratory tract infection and diarrhea.
