FDA Approves Ozanimod for Relapsing Multiple Sclerosis
The Food and Drug Administration (FDA) approved ozanimod (Zeposia; Bristol Myers Squibb, New York, NY) for the treatment of adults with relapsing forms of multiple sclerosis (RMS), including clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease. Ozanimod, an oral medication taken once daily (0.92 mg), is the only approved sphingosine-1-phosphate (S1P) receptor modulator that offers individuals with RMS the ability to start treatment without genetic testing or required first-dose observation. An up-titration scheme should be used to reach the maintenance dosage of ozanimod, because a transient decrease in heart rate and atrioventricular conduction delays may occur.
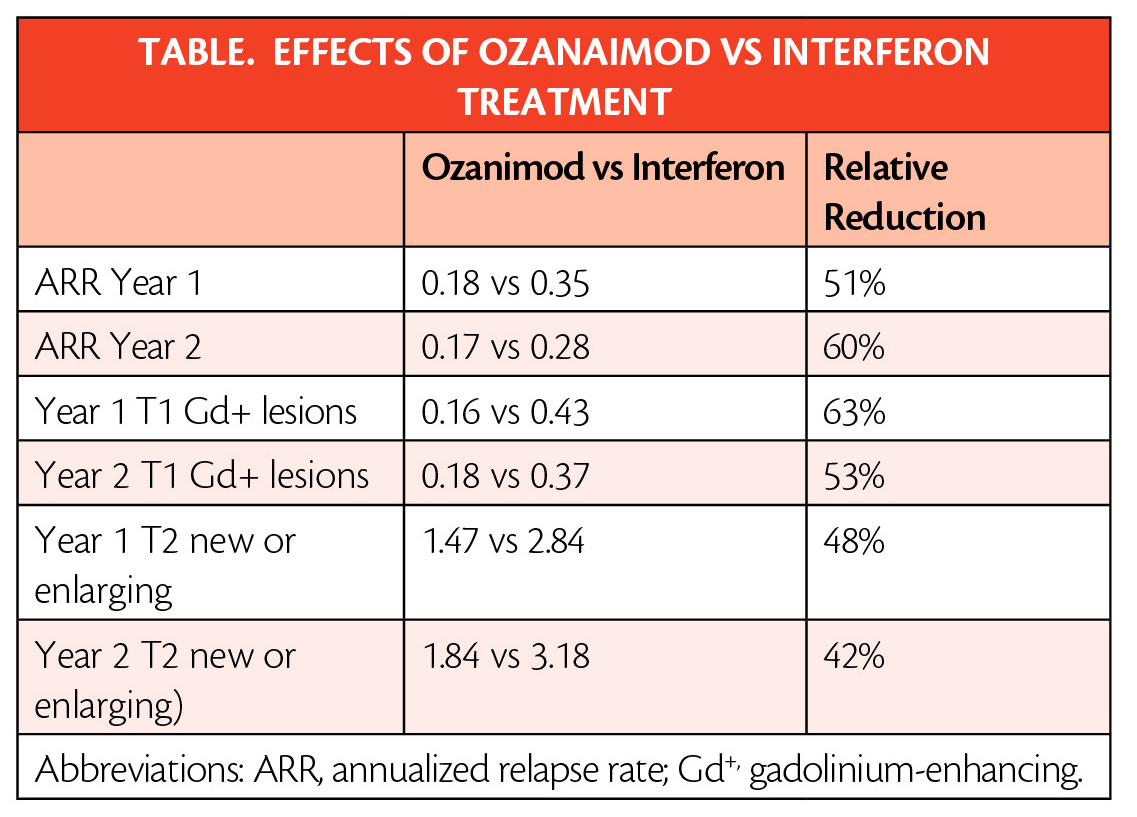
The approval is based on data (Table ) from the largest pivotal, head-to-head RMS studies with an active comparator to date: the randomized, active-controlled phase 3 SUNBEAM (NCT02294058) (safety and efficacy of ozanimod vs interferon in RMS) and RADIANCE (NCT01628393) (safety and efficacy of the selective sphingosine 1-phosphate receptor modulator ozanimod in RMS) Part B clinical trials of more than 2,600 adults.
“With the FDA approval of Zeposia, appropriate patients with RMS will have another oral treatment option with meaningful efficacy to help address the disease’s hallmark relapses and brain lesions,” said Samit Hirawat, MD, chief medical officer, Bristol Myers Squibb. “Zeposia has substantial clinical potential, and we are well positioned with our heritage in transformational science to ensure this innovative compound ultimately benefits as many patients as possible.”
“Treatment for relapsing forms of multiple sclerosis is critical to address this devastating neurological disease. I’m excited, with the introduction of ozanimod, I will have a new oral option to offer my RMS patients that has demonstrated efficacy and safety,” said Bruce Cree, MD, PhD, MAS, professor of clinical neurology, University of California San Francisco (UCSF) Weill Institute for Neurosciences and clinical research director, UCSF MS Center.
Ozanimod demonstrated acceptable safety and tolerability in the phase 3 sunbeam and radiance part B trials. The most common adverse reactions (incidence ≥4%) were upper respiratory infection, hepatic transaminase elevation, orthostatic hypotension, urinary tract infection, back pain, and hypertension. Ozanimod is contraindicated in participants who in the last 6 months experienced myocardial infarction, unstable angina, stroke, transient ischemic attack (TIA), decompensated heart failure requiring hospitalization, or class 3/4 heart failure; participants who have a presence of Mobitz type 2 second or third-degree atrioventricular (AV) block, sick sinus syndrome, or sinoatrial, unless the participant has a functioning pacemaker; participants with severe untreated sleep apnea; and participants taking a monoamine oxidase inhibitors.
The following tests are required before starting ozanimod: complete blood count including lymphocyte count (within 6 months or after discontinuation of prior multiple sclerosis (MS) therapy), an ECG to determine whether preexisting conduction abnormalities are present, a recent liver function test (within 6 months), and consideration of current and prior medications, including vaccinations. For individuals with a history of uveitis or macular edema, an ophthalmic assessment is required.
