FDA Accepts New Drug Application of Trofinetide for Rett Syndrome Treatment
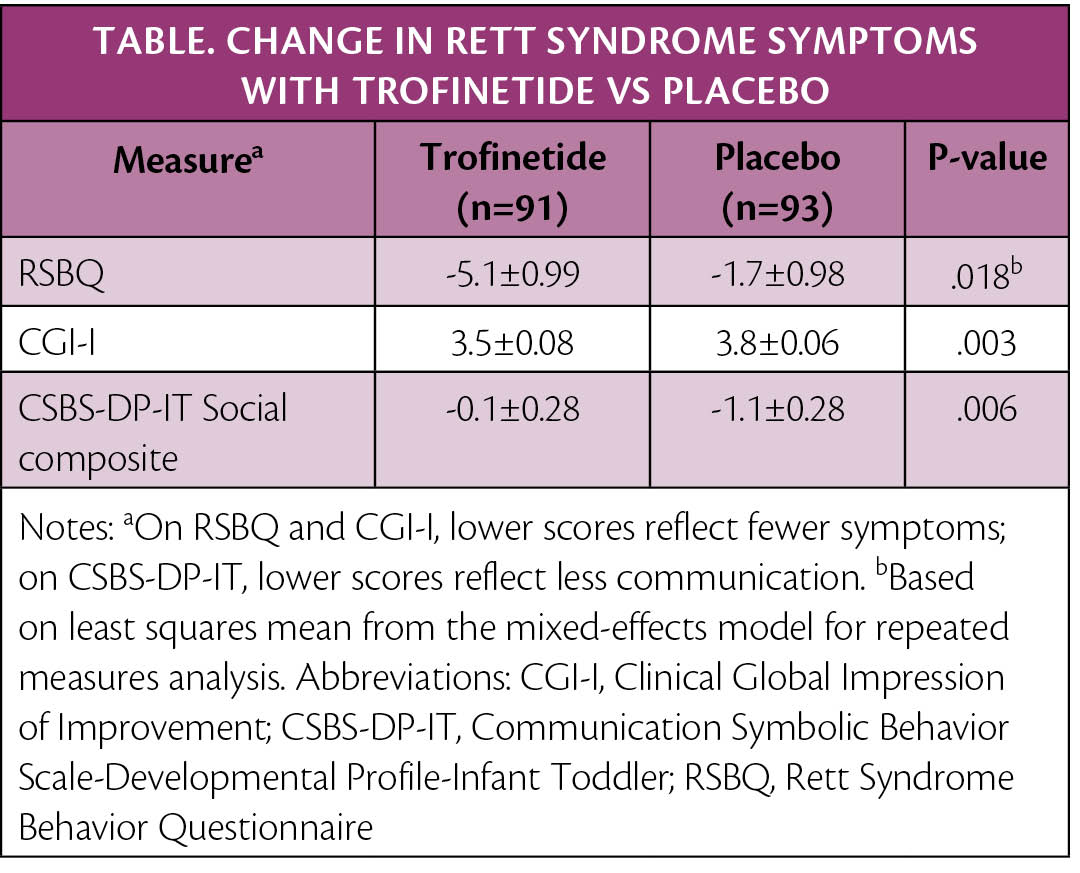
The Food and Drug Administration (FDA) has accepted filing of a New Drug Application (NDA) for trofinetide (Acadia Pharmaceuticals, San Diego, CA) for the treatment of Rett syndrome. Support for the application comes from the pivotal phase 3 Lavender study (NCT04181723), the results of which were reported at the American Academy of Neurology in April, 2022. As shown in the Table, treatment with trofinetide vs placebo resulted in statistically significant improvements, as measured with caregiver and clinician symptom-rating scales.
“We’re pleased that the FDA has accepted our NDA filing and we will be working closely with them to facilitate completion of the review in a timely manner,” said Steve Davis, Acadia’s chief executive officer. “If approved, trofinetide will be the first drug available for the treatment of Rett syndrome, a rare and devastating condition for patients and their families. This milestone reinforces Acadia’s ongoing commitment to advancing research into high unmet needs in disorders affecting the central nervous system.”
“Rett is a complex disease that can present with a diverse array of symptoms. In clinical trials, trofinetide demonstrated a significant improvement in a range of Rett syndrome symptoms,” said Jeffrey L. Neul, MD, PhD, Annette Schaffer Eskind chair and director, Vanderbilt Kennedy Center, professor of Pediatrics, Division of Neurology, Pharmacology, and Special Education, Vanderbilt University Medical Center and phase 3 Lavender study investigator. “We look forward to the FDA’s review of this submission and the prospect of having access to the first approved treatment for Rett syndrome.”
Participants were identified as girls and women age 5 to 20 with genetically verified Rett syndrome who had not experienced developmental regression in the 6 months before screening or a change in seizure pattern in the 8 weeks before screening.
The most common treatment-emergent adverse events with trofinetide were diarrhea, which was mostly mild or moderate, and vomiting.
